| Pages:
1
..
60
61
62
63
64
..
81 |
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Cheer up, there is life after fast foods. My head manager got as far as their management, before becoming a vegetarian and full time pyro and physical
theatre/lighting professional. He is much happier now, and can tell you amusing and interesting facts about McDonalds, like "in a food fight, the guy
with the tartar sauce gun ALLWAYS wins".
May I direct your attention to this thread?
strategies in designing ideal explosives thread
[Edited on 8-2-2018 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by Bert  | Cheer up, there is life after fast foods. My head manager got as far as their management, before becoming a vegetarian and full time pyro and physical
theatre/lighting professional. He is much happier now, and can tell you amusing and interesting facts about McDonalds, like "in a food fight, the guy
with the tartar sauce gun ALLWAYS wins".
May I direct your attention to this thread?
strategies in designing ideal explosives thread
[Edited on 8-2-2018 by Bert] |
Nice thread, looks like its exactly what I want.
Regarding mc donalds, I don't think I wanna be working there much longer. There is so much nastiness in the food that it makes me sick. I used to work
in the kitchen, but now I'm just the bridge between the girl taking orders and the people in the kitchen, much better. I still feel sick when I smell
the fat coming from the burgers and other stuff.
Oh, hello!  |
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
Hello , id like to ask what is the best way to dry some KCLO4. Its stored for a long time and has enough humidity
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Potassium perchlorate isn't hygroscopic in my experience. I have never needed to dry commercially produced material.
Where did this perchlorate come from?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I have never needed to dry it either. Are you sure its not just clumping, I had a batch of perc once that really liked to clump and the stuff I have
now is quite free flowing.
[Edited on 9-2-2018 by greenlight]
Be good, otherwise be good at it 
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Feedme, your salt is not pure KClO4. You try test in water by 15 Celsia. KClO4 solubility = 1,5g /100ml H2O. NaClO4 solubility = 209g /100ml H2O. You
can try your KClO4, for example 3 grams get into 100ml H2O. If will all solubled, is not pure KClO4. But the mix between both. Or even some else
compounds can be in this.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
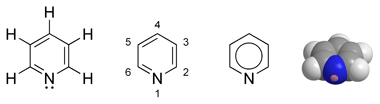
Can you nitrate Pyridine?
I was wondering if it would be possible to knock off all of those hydrogen molecules and place NO2 groups there by means of an aromatic electrophilic
nitration. I don't know if pyridine would oxidize or not, wikipedia doesn't say much about it, but it could be sulfonated first and the respectively
carbon positions in which the RSO3− groups were attached would get replaced by the NO2.
Oh, hello!  |
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
IIRC, the direct nitration of pyridine is possible only using extremely high temperatures due to the pyridine (or pyridinium ion) not being very
activated. Nitration of pyridine-n-oxide is more easy, producing the 4 nitro isomer (Attachment).
https://www.oc-praktikum.de/nop/en/instructions/pdf/1004_en....
From what I read, it is not possible to nitrate even the n-oxide to trinitropyridine n-oxide, although seems possible using an alternative synthesis
from dinitroethanol:
Attachment: 2,4,6-Trinitropyridine and Related Compounds,.pdf (460kB)
This file has been downloaded 620 times
-----------------------------------------------------------------------------------
Here is an idea I have been toying with for some time, any thoughts about this?
The piria reaction uses bisulfites at a pH of about 6-6.5 to reduce a nitrogroup to an amine. In some cases, a sulfonic acid group is introduced to
the nucleus as well, desribely for nitrophenols in meta position.
I was therefor wondering what products would result if the piria reaction would be applied to picric acid instead? Bisulfite is a pretty decent
nucleophile (Couldn't find any measure of this compared to CN-
or HS- though), would it be able to attack the picric in meta (position 3) and introduce a sulfonic group upon autooxidation of the presumed
meisenheimercomplex? When I did a quick test, adding a spatule of sodium picrate to some bisulfite solution at a pH of 6.5 and heated it slightly,
very quickly a very strong red-orange colour was produced. So it seems something is happening at least. When some HCl was added a dark orange brown
developed. Might be interesting since, unlike thee reaction of picric with HS- and CN-, the reaction with bisulfite has never been characterized
AFAIK.
Attachment: Piria reaction.pdf (832kB)
This file has been downloaded 568 times
[Edited on 14-2-2018 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Bisulfite is one of the reducing agents I had predicted would likely produce a picramate from a picrate. It would probably work even better with an
added mole equivalent of NaOH to convert the bisulfite to the normal sulfite.
[Edited on 2/15/2018 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@ Bert No it was in reply to nitro-genes describing a red orange color from reaction of sodium bisulfite and sodium picrate, which probably produces
sodium picramate.
|
|
|
Bert
|
Thread Split
15-2-2018 at 14:24 |
Vomaturge
Hazard to Others
  
Posts: 286
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
The compound phenol sounds like it should be an alcohol. Apparently, it can form esters, like phenyl acetate:
https://en.m.wikipedia.org/wiki/Phenyl_acetate
So why doesn't the hydroxyl group on phenol/picric acid get replaced with a nitrate group during a nitration? My guesses would be
1. Because phenol "carbolic acid" and picric acid are acids, and thus do not become esters easily.
2. Because it turns to picric first, and the nitro groups take up too much space to allow HNO3 in to react with the hydroxyl group.
3. To make phenol acetate, you apparently need acetic anhydride. So does that mean that TNP mononitrate might possibly be formed using dinitrogen
pentoxide? Thanks.
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
The hydrogen on the hydroxyl group of phenol is slightly acidic due to the electron delocalization of the benzene ring, so a nitration on the hydroxyl
group is similar to trying to chlorinate the hydroxyl of acetic acid with HCl; a strong acid won't react with a weak acid.
The philosophy of one century is the common sense of the next.
|
|
|
Texium
|
Thread Split
8-3-2018 at 06:05 |
EdsonEGDN
Harmless

Posts: 7
Registered: 13-3-2018
Member Is Offline
Mood: No Mood
|
|
AN Based explosives
Hello, I think some of you may have seen some of my tests on YouTube, I study chemistry and I have a good knowledge in the field of Explosives.
I am here for suggestions of compositions based on Ammonium Nitrate ( AN ) , I have tested many of them
ANNMAl
Ammonal Versions ( With Mg , Sulfur , charcoal powder , sugar )
AN + Mg powder
ANNAPHAL ( AN , Naphthalene and aluminum Powder )
ANFO versions ( ANFO + Al , ANFO + 2% Zn powder , ANFO + C )
AN + Ethylene glycol
AN + Glycerin
AN + Sulfur
AN + C
ANUNAL ( AN , Urea Nitrate and Aluminum powder )
AN + TATP
AN + Hexamine and with Al powder
AN + sugar
AN + Urea
AN + Nitric Esters + Al powder
AN + H2O2 Water gel ( Stable Mix )
The top 10 better compositions are 1° AN + Nitric Esters + Al , 2° ANNMAl , 3° AN +H2O2 Water gel , 4° AN + TATP , 5° ANNAPHAL , 6° ANUNAl , 7°
Ammonal + C , 8° AN + Ethylene glycol , 9°ANFO + Al and 10° AN +Hexamine .
Tell my any other Composition AN based ((( without Nitric Esters !!! or Nitrocompounds ))))) !
[Edited on 13-3-2018 by EdsonEGDN]
[Edited on 13-3-2018 by EdsonEGDN]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
If you have advanced knowledge of explosives, why would you need to ask?
|
|
|
EdsonEGDN
Harmless

Posts: 7
Registered: 13-3-2018
Member Is Offline
Mood: No Mood
|
|
It is always welcome new suggestions, of course someone with advanced knowledge about tested something that I have not had in mind yet and can be
quite interesting for me .
I have many ideas but I want to hear what others have in mind, who knows is something I never thought of before, with Ammonium Nitrate the possibility
is almost infinite!
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
I seem to remember some axt(number I don't remember) on at least two publicly available online platforms demonstrating ANNMSA (SA=sulfuric acid) and
IIRC ANNMAlSA (no idea how the Al did not react with the acid, maybe my memory is failing me) had a much improved vod as witnessed in a metal plate
test. These mixtures preformed slightly less than NANM (NA=nitric acid) which these previously mentioned mixes make except they contain inert ammonium
sulfate.
On what basis did you make your top 10 list?
|
|
|
EdsonEGDN
Harmless

Posts: 7
Registered: 13-3-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sigmatropic  | I seem to remember some axt(number I don't remember) on at least two publicly available online platforms demonstrating ANNMSA (SA=sulfuric acid) and
IIRC ANNMAlSA (no idea how the Al did not react with the acid, maybe my memory is failing me) had a much improved vod as witnessed in a metal plate
test. These mixtures preformed slightly less than NANM (NA=nitric acid) which these previously mentioned mixes make except they contain inert ammonium
sulfate.
On what basis did you make your top 10 list? |
In the power ( Brisance ) and noise of explosions in my tests, I like to compare so I'm sure of the power of each one to another Composition .
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
How about simple ANFO? AN/ kerosene, Diesel or heating oil. Soybean oil is said to work also but requires a long soak time(1 week) there are several
AN/ hexamine combinations too. Also adding laundry powders to some compositions can help the power. Have you read this?
https://archive.org/details/Kitchen_Improvised_Fertilizer_Ex...
There’s a few in there you may be interested in.
|
|
|
Bert
|
Threads Merged
13-3-2018 at 17:08 |
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Ammonium nitrate/gum spirits of turpentine (old fashioned turpentine, distilled from pine stumps, etc.)
Ammonium nitrate/DMSO or other fuels capable of quickly and completely dispersing through solid crystals of AN.
Ammonium nitrate/trichloroethylene
Ammonium nitrate dissolved in water with a fuel such as ascorbic acid, erythorbic acid, alginic acid or their salts and then dried.
Ammonium nitrate/hydrazine (Yo, astrolite?)
Ammonium nitrate/amine, such as dimethylamine, ethylamine, etc.
|
|
|
EdsonEGDN
Harmless

Posts: 7
Registered: 13-3-2018
Member Is Offline
Mood: No Mood
|
|
Nice ! Are you the guy from Canal AllChemystery? 
[Edited on 14-3-2018 by EdsonEGDN]
|
|
|
EdsonEGDN
Harmless

Posts: 7
Registered: 13-3-2018
Member Is Offline
Mood: No Mood
|
|
I am going to try in next week intermolecular mixtures, AN samples with ammonium benzoate, benzoamine, benzonitrile, ammonium acetate, ammonium
phenolate and mainly ammonium thiocyanate it can give directly into the molecule Oxidant of AN the energy and reactivity it needs to ensure high VOD .
|
|
|
Bender84
Harmless

Posts: 32
Registered: 24-3-2016
Member Is Offline
Mood: No Mood
|
|
Gellatinizing power of NC
Hello World,
Can someone explain to me (or point me to a source where I can find such information) what are the critical parameters that define the gelling power
(if used with NG/ EDGN mixture as the "solvent") of nitrocellulose and how - if at all - is this characteristic related to viscosity of the NC
solution in e.g. acetone (i.e. is it justified to asses that high viscosity NC will give stiff gel? Or these properties run parallel but are not
directly related, so one cannot predict the gelling power based on the viscosity of the solution)? I really couldn't find any in depth info about the
gellation power of the NC beside the information given in Naoum's book.
Thanks in advance,
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Bert, I think Edson is just like me, he is so overcome by passion that he asks others because he wants no stone unturned, and I admire him for that.
Edson, I am sending you a U2U.
|
|
|
EdsonEGDN
Harmless

Posts: 7
Registered: 13-3-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MineMan  | Bert, I think Edson is just like me, he is so overcome by passion that he asks others because he wants no stone unturned, and I admire him for that.
Edson, I am sending you a U2U. |
U2U Answered
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
How hard is it to concentrate 53 % nitric acid to azeotropic concentration (68 %)?
I don't know how to set up my distillation apparatus and the fractionating column for this specific procedure.
Oh, hello!  |
|
|
| Pages:
1
..
60
61
62
63
64
..
81 |