| Pages:
1
..
4
5
6
7
8
..
13 |
chloric1
International Hazard
    
Posts: 1146
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Well, I have not been able to venture to the hardware store lately and my daughter has chicken pox. So homelife is rather demanding. Just enough
time to look into this forum a little here and there. Not to menton weather problems. Tornados n Jan/Feb? Whaaaat the hell?!  
I have the current density calculation for my first SnO2 attempt figured out. Unfortunately, I do not have the precision power supply with current and
voltage limiting like Xenoid has. I will fill everyone ASAP! I need to get on this because I know Xenoid is dying to know about nickel-cobalt spinel
mix.
[Edited on 2/5/2008 by chloric1]
Fellow molecular manipulator
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
elementary wattsome
On the preceding page I proposed that possibly an incoloy
sheathed hot water heater element might be modified as
an inexpensive heating element for a 2" square tube tube furnace .
I ordered one and have gradually gently opened the original bends
to reshape the element to a larger dimension .
Still have some final cambering of the element
to finalize the fit , but it looks like it may work out okay .
The calrod is 5/16" diameter instead of the 3/8" I was guessing before having the part in hand .
Here's the before modding Camco #04963 , 240V 5500W

and here's after modding

The length between the outside of the single hairpin to the
outside of the double hairpin is 11 1/2 inches . At 120 V
the element should produce something in slight excess of 1375W , which in an outside insulated 2 inch square tube
should get plenty hot enough for baking any anodes and
will probably go fine to 650C - 1200F as a comfortable limit .
The terminal block turned out to be polypropylene and a heat gun softened it up
enough that it pulled away in a couple of chunks easily .
The next step was opening up the double hairpin bend at the extreme end gradually until there was about 3 inches clearance at the single hairpin tip
where it was folded close to the parallels near the mounting plate as manufactured .
The mounting plate was separated using a vise and three separate cuts with a hacksaw , the first a straight cut with the blade at normal angle making
a cut from the backside of the folded section , the cut about a third of the distance through the plate . Then the plate was flipped over and
the blade was placed at the 60 degree side angle position
in the hacksaw frame , and a similar depth cut was made
from the folded hair pin side . Then the element was flipped
vertical and rotated 90 degrees in the vise , the blade was loosened and put between the elements where they enter
the mounting plate on the hot side , and with the blade still
at that side position used in the cut before , the middle third
remaining was cut completely through , intersecting the two
previous cuts to form a single cut , separating the plate .
Where there's a will there's a way 
I haven't yet tested the element to see if it has withstood the very gradual reshaping ,
but supposedly calrods can be bent okay before they are put into service . After they have been fired up , the heating element inside embrittles so
after firing they cannot be reformed . Therefore I am not going to energize the element until it is finished mounting in the tube whereafter it will
not be moved , except by its own thermal expansion and contraction within mountings which will allow that slight free movement .
[Edited on 9-2-2008 by Rosco Bodine]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Rosco, I've bent some heating elements scavenged from a toaster oven, of a similar type, and they withstood going from straight rods to spirals just
fine. I even cut one, and the resistance wire inside is actually wound in a spiral inside the tube (packed in whatever the hell that white crap is).
The elements I abused were most definitely used when I performed my "surgery".
Haven't been messing with anodes for the last week, I'm letting some SnCl2 I made dry out so I can make some of that oxidative soak solution. I've
been thinking of lining my graduated cylinder with a plastic bag or something similar so that I don't permanently coat it with SnO2.
Interestingly, on another tangent, I dropped one of my mystery non-lead sinkers into some HCl, and I get a bright piss-yellow solution, with black
scum floating on top. Any idea what metal this would be?
[Edited on 10-2-2008 by tentacles]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I don't know unless it is a thermoplastic adhesive used in the compression molding of the bismuth powder . Because
of the brittleness of bismuth I think they used that method
for manufacture to make the sinkers less prone to shattering on impact . They still break if you place them in a plastic bag and smack them sharply
with a hammer . Melting the material first is what I was going to do to make sure I got a clean metal . I haven't tried to process any of mine yet
.
[Edited on 10-2-2008 by Rosco Bodine]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
The bismuth sinkers I already melted, these are the ones I picked up at walmart, labeled simply "Non-lead Dipsey Bass Sinkers". They ARE quite
brittle, and hard. I drilled into one a bit and it was brittle and screechy but not terribly hard to drill. I'm thinking these must be iron, and
possibly they are coated with some kind of oil, wax or finish to prevent oxidation. I have not tried melting one yet.
My Bismuth sinkers seemed to be simply cast bismuth, there was no vapor or smoke when I melted them. I dripped the metal into cold water to produce
nice fragments, and it made some interesting shapes when it hit the water.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
BTW , nice to know that somebody is listening about the
sol-gel deposition and baking technology which I have been trying to reveal has applicability here for coating anodes .
It hasn't been stated openly as such in any of the literature
that doped tin oxide is a clathrate , but take my word for it  , that is
precisely what doped tin oxide is , that is
precisely what doped tin oxide is  . And I have tried to break that news
somewhat gently . And I have tried to break that news
somewhat gently  in another thread with a reference to the hundred year old
discovery of this clathrate structure by a Dutch chemist , in another thread with a reference to the hundred year old
discovery of this clathrate structure by a Dutch chemist ,
http://www.sciencemadness.org/talk/viewthread.php?goto=lastp...
It seems van Leent , like some other scientists of that era before the mechanism of inclusion compounds had been elucidated , were creating clathrates
and puzzled by them ,
for not really understanding what they had , other than it was a genuine curiosity  , an anomalous material which , an anomalous material which
seemed to be a compound of some sort but didn't behave
like usual compounds in every way .
The intermediate hydrosol gel formation which is thermally unstable and forms a mixed matrix with the included , or caged dopant material , in an
expanded water containing lattice structure , which collapses on dehydration by heating ,
is a *classic* clathrate formation mechanism which I certainly do recognize for what it is  . And the polycrystalline inclusion compound which results is a "solid solution" , but likely at the point of saturation with dopant , it
is also more precisely an inclusion compound or in other words a clathrate . . And the polycrystalline inclusion compound which results is a "solid solution" , but likely at the point of saturation with dopant , it
is also more precisely an inclusion compound or in other words a clathrate .
It's time for a diva break 
http://www.youtube.com/watch?v=faKFcfytlxU
[Edited on 10-2-2008 by Rosco Bodine]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by tentacles
Haven't been messing with anodes for the last week, I'm letting some SnCl2 I made dry out so I can make some of that oxidative soak solution. I've
been thinking of lining my graduated cylinder with a plastic bag or something similar so that I don't permanently coat it with SnO2.
[Edited on 10-2-2008 by tentacles] |
Same here!
I have a beaker half-full of concentrated SnCl2 solution, I know the approximate molarity from the amount of tin I dissolved (which took forever). Is
there any point in trying to crystallise it, is it straight forward, any tricks! I noticed a thin film, in the bottom of a beaker, crystallised nicely
on evaporation!
I was thinking of doing the oxidative soak in a test tube, upright in a beaker of hot water on a hot plate. The temperature has to be kept at 60 oC
+/- 5 oC, (333 oK, Table 2. Page 1242). Film is only 100nm (0.1 um) after 24 hours, so may require several days for a reasonable thickness, eg. 0.5
microns. This is reduced by 30% when pyrolised. I will also be adding some Bi to make it conductive.
I have made some Bi(NO3)3 went OK except for the clouds of NO2, the reaction is quite vigorous with 68% HNO3. I had to take the beaker down to the
bottom of the garden! I had to keep adding bits of Bi and it still kept bubbling, solution was yellow/green from dissolved NOx, then overnight the
whole lot turned to colourless crystals.
Bi is only slightly attacked by HCl and the chloride is normally made by the action of conc. HCl on Bi2O3. I have dissolved pottery Sb2O3 in HCl and
got a pale yellow colour!
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Xenoid: So if you are doping the SnO2 with Bi, in an oxidative soak situation, what Bi compound are you adding to achieve this? Is a dopant required
for a sealing intermediate layer to be conductive? Does the application and subsequent baking of the SnO2 deposited by ox-soak over Co spinel provide
sufficient doping?
I wonder what would happen if the oxidative soak was tried using Bi(NO3)3 and SnCl2?
[Edited on 10-2-2008 by tentacles]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The initial sealing layer of SnO2 over the cobalt spinel can be pure SnO2 , since it will dope itself upon baking by diffusion
of the cobalt spinel from below .
However an oxidative soak deposited pure SnO2 layer can
also be doped by diffusion of a dopant from above , the
dopant also applied in the cold , and then the doped SnO2
developed on baking . Attached is an example for antimony doping , but other dopants could be applied similarly .
I still say that use of a Pytlewski polymer wetting agent as a
simple dip and dry treatment between coats should improve the results for any of these coating sequences .
Attachment: Antimony doping of tin oxide coatings prepared by the oxidative-soak coating method .pdf (325kB)
This file has been downloaded 1040 times
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by tentacles
I wonder what would happen if the oxidative soak was tried using Bi(NO3)3 and SnCl2?
[Edited on 10-2-2008 by tentacles] |
Yeah! I haven't quite worked that out yet! Adding a small quantity of Bi(NO3)3 or BiCl3 to the soak solution will result in bismuth subnitrate and
oxychloride respectively forming, which is undesireable. I may try multiple oxidative soak/ pyrolysis for the SnO2 with some Bi(NO3)3 pyrolysis
layers interspersed.
BTW Hubert is still running in the KClO3 cell, I will have to call it a day soon though, as I'm getting a bit sick of
it. It's been running continuously for 42 days (1008 hours) now. Every few days I scoop out KClO3 and add some more KCl (a bit like running a ginger
beer plant). The electrical characteristics are very, very slowly degrading. I think if you put on 20 coats of MnO2 and kept the current density to 50
mA/cm^2 it would just about run forever ... 
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Tin nitrate and bismuth nitrate might work .
According to US4272354 , Bi(NO3)3 and SnCl4 are workable together , and also BiCl3 and SnCl4 .
Here's another soak deposition method which is similar but involves only hydrolysis of the precursors which are already at the higher oxidation state
. From what I have understood
the pH range and concentration and temperature must be
in a narrow window for these deposition methods to work well , but then the conditions are also pretty specific for
other methods . If done right , a transparent well adhering
film is deposited , and if done wrong the result is a dusty deposit which wipes right off and doesn't stick at all .
[Edited on 10-2-2008 by Rosco Bodine]
Attachment: Electroless deposition of SnO2 and Antimony doped SnO2 films .pdf (505kB)
This file has been downloaded 983 times
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
The "white crap" inside heating rods is sintered (dead-burned) magnesia, which has a high thermal conductivity for an electrical insulator. It is also
extremely heat resistant.
Aren't those heating rods expensive? Won't a few selfmade NiCr wire coils inside a makeshift oven chamber made from aerated concrete blocks (Ytong),
which is how I built my first 1000°C tube furnace, do the job as well?
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Rosco, I read through that second paper on the Sb doping, and it sounds very much like an adjunct to the first. They use the same method to deposit
SnO2 film, then dope it by soaking in SbCl3 + HCl solution, then bake at 400C for 10 minutes.
Garage chemist: It's nothing more than a dry-fire capable water heater element. An oven element could also serve, I would think. I doubt you'd get
1000C out of these but for a lower temp furnace it should suffice, and they are definitely cheap, and durable.
|
|
|
microcosmicus
Hazard to Others
  
Posts: 287
Registered: 31-12-2007
Member Is Offline
Mood: spin up
|
|
| Quote: |
The "white crap" inside heating rods is sintered (dead-burned) magnesia,
|
When I've opened up heating rods, the insulator inside was liquid, so they
must have used some sort of oil to make a paste out of the magnesia.
I guess silicone oil was used but does someone know for sure? Since
these heating rods are intended for applications where the temperature
does not go too high, I presume that the oil would boil and make a mess
of the element if one heated it much beyond the usual operating temperature.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I was talking about heating rods that do normally operate without contact with any liquid, like the heating rods in a kitchen baking oven with grill
function. Those do get red hot (ca. 800°C) during normal operation, and do contain pure magnesia as the insulator and nothing else. I have never seen
or heard about rods that contain a liquid.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
| Quote: | Originally posted by Xenoid
BTW Hubert is still running in the KClO3 cell, I will have to call it a day soon though, as I'm getting a bit sick of
it. It's been running continuously for 42 days (1008 hours) now. Every few days I scoop out KClO3 and add some more KCl (a bit like running a ginger
beer plant). The electrical characteristics are very, very slowly degrading. I think if you put on 20 coats of MnO2 and kept the current density to 50
mA/cm^2 it would just about run forever ...  |
Have you tried running the Mn Oxide anode in a Perchlorate cell. Perhaps you have reported it and I missed it.
What (roughly) is the voltage accross cell now?
Cheers,
Dann2
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by dann2
Have you tried running the Mn Oxide anode in a Perchlorate cell. Perhaps you have reported it and I missed it.
What (roughly) is the voltage accross cell now?
|
Dann2, you may recall Gertrude and Mathilda, they did not perform particularly well in a perchlorate cell. They both had similar coats to Hubert!
Hubert started out (2nd day) at 3.6 volts / 2.0 amps (55mA/cm^2) and is now running at 4.4 volts / 2.0 amps. I've been running it at 2.0 amps most of
the time, except for several days in the middle at 3.6 amps, which seemed to do a bit of damage to the coating!
The cell has been on a stirrer / hotplate with constant stirring and temperature maintained at 42 oC. I could have run it hotter, which would have
improved the electrical characteristics with it being a KCl cell, but that temperature is what it stabilised at on the lowest setting, so I just left
it!
[Edited on 11-2-2008 by Xenoid]
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
@Xenoid
42 days and still standing, fantastic! Just imagine the crud you would have from an infinity of gouging rods. Have you been watching pH? You may have
mentioned it above, but it's difficult to sort out from your numerous posts.
Any idea of current efficiency? Should also be better than carbon, one would think.
Great work. Now for perchlorate...
Regards
Der Alte.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by tentacles
Rosco, I read through that second paper on the Sb doping, and it sounds very much like an adjunct to the first. They use the same method to deposit
SnO2 film, then dope it by soaking in SbCl3 + HCl solution, then bake at 400C for 10 minutes. |
There's simply some more evidence there that dopant addition can be done by diffusion on baking .
It would seem likely that BiCl3 could be used identically as is SbCl3 .
And Bi(NO3)3 can be applied as a 10% solution to form the oxide on baking , but the solution of Bi(NO3)3 must be dissolved in and kept acidic with
dilute HNO3 as it begins to hydrolyze immediately .
BTW , jpsmith123 had first posted one of the Bi related patents which I later found independently , I just ran across it while doing a search
http://www.sciencemadness.org/talk/viewthread.php?goto=lastp...
US4353790 indicates that Bi is useful as an interface on Ti substrate anodes , either plated on as the metal and oxidized on baking , or applied as a
10% solution of the nitrate directly to a Ti substrate and then baked to the oxide . In the patent , the Bi interface is used in conjunction with an
overcoating of Iridium and Iridium Oxide to produce anodes having twenty times the durability of Pt plated Ti anodes . Of course Bi in other patents
reportedly also performs well as a dopant for SnO2 and MnO2 .
CRC reports Bi(NO3)3-5H2O (mol.wt. 485.07) solubility as
42 grams in 100 ml acetone at room temperature , which would seem to make acetone
the solvent of choice for any dip coating scheme .
Bi(NO3)3 is reportedly unstable to heating even slightly warm , CRC says decomposition at 30C
with loss of 5 H2O at 80C which I assume is for the neutral salt in the absence of any stabilizing excess of HNO3 .
I would presume from this that neutral Bi(NO3)3 would be something then which you would want to crystallize out from dilute HNO3 ( maybe .5 to 1.5
molar ? ) in the cold and dry without any warming in the cold , looks like a refrigerator storage item . How stable is the acetone solution , I have
no idea . Interesting chart below also are double salts of
Bi(NO3)3 which might also be soluble in acetone , particularly
interesting is the Mn(NO3)2 , and the juxtaposition with some
other materials of interest like Co and Ni . It makes me curious about stannic nitrate as well .
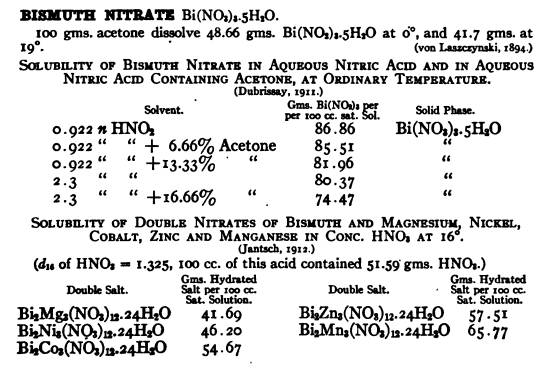
The above chart was excerpted from a book downloaded from here http://books.google.com/books?id=G5BLAAAAMAAJ&pg=PA151&a...
[Edited on 12-2-2008 by Rosco Bodine]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
@ Rosco
We still seem to be running around in circles, it would be really nice if some kind soul could make a list of all the
patents quoted in the various anodes threads, along with a descriptive title.
US4272354 is one of the main patents I've been looking at in terms of bismuth, I believe this is the US patent equivalent of the WIPO International
Patent WO 79/00842 that you and chloric1 got excited about a while back. It has some of the same diagrams.
Bi(NO3)3 is easy and straight forward to make, and the moist (with dilute HNO3 left over from the dissolution) crystals are quite stable in the fridge
at least (see post up the page).
I've done some solubility checks;
1) I put a small spatula load of crystals in the bottom of a test tube, added some acetone - instant white ppt. of bismuth subnitrate (BSN)
2) I put a small spatula load of crystals in the bottom of a test tube, added some isopropyl alcohol - instant white ppt. of BSN, but very voluminous,
and stays in suspension!
3) I put a small spatula load of crystals in the bottom of a test tube, added some 68% HNO3, a ml or so, enough to easily cover the crystals, they
partly dissolved!
4) I added an equivalent amount of H2O to the above (3) ALL the crystals dissolved and the solution remained clear!
5) I repeated (4) about 10 times and the solution remained clear.
6) By this time the test tube was about half full of solution, so I tipped it into a small beaker and added water to double the volume, solution was
still clear. At this stage I checked the pH it was still way down at about 1.
7) I put some of this dilute solution in a test tube and added an equivalent amount of isopropyl alcohol, a white hazy layer of BSN formed at the
interface, when I shook it up the white disappeared and the solution cleared.
8) Repeated (7) with acetone and got same result.
So in summary, I don't think there is any problem making Bi(NO3)3 solutions for pyrolysis with, say Mn(NO3)2 as I have these solutions acidified with
HNO3 anyway. The important thing to do is mix the Bi(NO3)3 with a little HNO3 first, before mixing with other solutions, and keep the pH (very) low.
[Edited on 12-2-2008 by Xenoid]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
That's strange about the acetone . Many references may be in error , perhaps in translation and it is acetic acid that
is the solvent , not acetone ???? But they do say acetone .
Maybe it needs a trace of nitric acid at the start of solution .
Maybe this was covered before , but I don't remember it .
I just excerpted the Bismuth Nitrates related material from Gmelin . I also ran across a note there or somewhere else that acetic acid added to a
nitric acid solution of Bi(NO3)3 would greatly reduce the tendency to precipitate the basic nitrate upon dilution with water. Also there is a mention
in Gmelin that if a Bi(NO3)3 solution is very gently evaporated
at a temperature low enough that evolution of nitrogen oxides and loss of nitric acid does not occur , that a concentrated uncrystallizable syrup
remains .
[Edited on 12-2-2008 by Rosco Bodine]
Attachment: Bismuth Nitrate Gmelin.pdf (312kB)
This file has been downloaded 726 times
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Oxidative Soak
@Rosco - this is for you!
I've just started another anode, this one is destined for a perchlorate cell. I have put on 4 coats of Co3O4 using the same procedures as outlined in
earlier posts. I am following this with an oxidative-soak-coat of SnO2. Over the top of all this I plan to do
several coats of Bi doped MnO2. If this coating scheme shows no improvement over the earlier MnO2 coated perchlorate anodes, I think I will give up on
MnO2.
I have used the solution concentrations suggested in the paper; .01M SnCl2 and .05M KNO3. The soak solution was made up by pippeting .5 mls of 2M
SnCl2 stock solution and 5 mls of 1M KNO3 stock solution into a 100 ml volumetric flask. About half this quantity is being used for the soak.
My setup is shown in the attached image. The Co3O4 coated Ti rod is resting in a 25 mm diameter test tube containing the soak solution,which in turn
is supported in a large beaker of water on a hotplate. The temperature is coming up to 60 oC at the lowest hotplate setting. I will probably let the
soak run for 48 hours.
I think I will call this anode Gunther (brave warrior).
Update: Temperature has stabilised at 59 oC. Unfortunately, after only an hour a little whitish floc has formed in the test tube, and the solution is
a little hazy. This is not supposed to happen!
Edit: Hmmm.. after 2 hours some of the floc has settled out, it's a dirty grey brown colour, may be just due to
impurities in the water.
[Edited on 12-2-2008 by Xenoid]

|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Putting some theory to the test huh ? 
How was the wetability of the spinel without a mixed valency polymer pretreatment ,
did it seem to wet out fine by the SnCl2 oxidative soak solution ?
I'm thinking all you should see as a result is a change
in the sheen and color, like a clear coat or perhaps a
slightly hazy clear coat , and a more visible glassy appearance to the coating after baking .
With four coats of spinel there , it may soak up the SnO2 like
a sponge , so it may take a couple of repetitions .
It may not even take four coats of spinel for the interface
before the SnO2 , but only one or two may do it .
The SnO2 should be followed by Bi2O3 doped SnO2 .
A great time to try stannic nitrate there , or even here
if the oxidative soak doesn't seal and build thickness
rapidly . IIRC there is a point where the thickness
of the hard deposited material limits out , per coat ,
and thereafter a bulk precipitation rather than a controlled
deposition just deposits the usual slime .
Don't get in too big a hurry with the Bi2O3 - MnO2 ,
save that for last .
BTW , in the article it was reported that slight turbidity was
normal in the first prepared solutions even using reagent grade materials and distilled H2O ,
so they held the solutions at 80C for three hours and then filtered them before use .
Looks like you should prop open that watchglass cover slightly ,
to allow the solution to have a very limited access to air so it can breathe a little .
Something which has been an idea for awhile concerns the
stannous nitrate which was described in the antimony and tin nitrate thread
http://www.sciencemadness.org/talk/viewthread.php?goto=lastp... .
Evidently the stannous nitrate solution is only stable at
lower temperatures and at higher temperatures it hydrolyzes and precipitates
a hydrated stannous or stannic oxide .
This has made me wonder what might happen
if a relatively warm anode say at 95C , like would be for
the case of an anode having a baked spinel , then dipped
and blow dried mixed valency polymer , was plunged into
a cool solution of stannous nitrate . I wonder if the the
heat loss there at the anode surface would result in a flash deposit of an adherent film
of hydrated SnO2 , derived from local thermal decomposition of the stannous nitrate solution
immediately adjacent the hot surface . If it was adherent ,
then it would be simple to build thickness . You could have
a beaker of stannous nitrate sitting in an ice bath , and
a heat gun handy for drying and warming up the anode
and just do sequential dips to build a thickness , and then bake at a higher temperature
to sinter the material . This might be thought of as an oxidative quench deposition method 
with the Ti blacksmith dipping the "not so hot but pretty warm anode" into the "special water"  , ,
to flash coat an oxide .
Also stannous nitrate might work as an oxidative soak deposition material
which could operate at room temperature as opposed to the 60C for the SnCl2 .
The stannous nitrate might set about an auto deposition simply by removing it from the refrigerator ,
diluting it and exposing it to air . And it would also likely be compatable with Bi(NO3)3 .
Anyway this is all purely hypothetical but might be worth experimenting at some point .
[Edited on 13-2-2008 by Rosco Bodine]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Rosco: The wetability seems very good, when I pulled my anodes out of the solution to filter it, they are completely wetted with no beading or running
of the solution. When you say mixed valency polymer solution, to what are you referring exactly?
Xenoid: The paper does mention letting the solution sit 3 hours at temp, and then suction filtering it. I think the ppct is normal. Mine went
hazy-clear-hazy after I filtered a second time (about 2 hours) and there seems to be no new ppct after filtering after 4 hours. My ppct was milky
white. I used .55-.6g SnCl2 and 1.2g KNO3 (I think, I'd have to check my math) in 250ml hot water. I picked up a cheap super tall shot glass to use
for my oxidative soaking. I'd rather SnO2 coat a $2 glass than my good cylinders!
I've been working on my Bi(NO3)3, this time in the freezer with ~70% nitric. It's still just giving me a white ppct? I distilled more nitric last
night, this batch is 1.51 sg so ~97% so I can try that. Also made some acetic acid while I had the distillation rig out.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The mixed valency polymer is a partially hydrolyzed and partially reduced Sn (+IV) soluble compound cross linked
with an Sn(+II) or some other substituted metal .
US3890429 is the patent
here's where it first came up
http://www.sciencemadness.org/talk/viewthread.php?goto=lastp...
and here's the link for the patent
http://www.sciencemadness.org/talk/viewthread.php?action=att...
Attachment: US3890429 STANNIC_OXIDE_POLYMER_Film Wetting Agent.pdf (399.45 KiB)
|
|
|
| Pages:
1
..
4
5
6
7
8
..
13 |