| Pages:
1
..
4
5
6
7
8
..
60 |
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Esplosivo, it's Ca(H<sub>2</sub>PO<sub>4</sub> <sub>2</sub> that is needed. <sub>2</sub> that is needed. 
Off topic, but solder composition methinks depends on your locale. Lead-tin solder has not yet been phased out in some territories. 
So you may be both correct. (Personally though, I have yet to see zinc-tin solder.)
evil_lurker's idea sounds good, but just to avoid the odoriferous carbon disulfide, are there substances where white phosphorus is soluble and
red phosphorus is not?
sparky (^_^)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Conversion of WP into RP via light proceeds incredibly slow, it is useless as a preparative method.
A better method to get out the phosphorus is to fill water into the pipe, warm it to 50°C, let it cool again and take the phosphorus blob out with
pliers.
RP is insoluble in all solvents, BTW.
[Edited on 14-4-2005 by garage chemist]
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Red phosphorus is soluble in phosphorus tribromide, also the reaction of white phosphorus with light is appreciable, even a solid hunk of white
phosphorus will convert to red readily in the presence of light. I have some that I could show you that has turned somewhat rapidly, check my book
project under phosphorus at the end and I have a little picture.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
The only other half arsed decent WP solvent I can find is benzene. WP is soluable in it up to 1 g/35 ml. WP is very soluable in carbon disulfide...
1g/0.8 ml... 1 liter of it would go a loooong way.
Phosphorus tribromide is not a good candidate for a solvent either. The RP is soluable in it as well as the WP making the final product hard to
separate.
As far as sunlight turning the WP in RP, I think it might be worth a shot to expose it to a concentrated UV radiation source. A 400 watt mercury vapor
lamp that has had the outer glass bulb broken off will put out a crapload of UV radiation. Coupled with a glass container of proper UV conductivity,
it would be interesting to see what would happen.
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Yes, it takes a lot of benzene to dissolve appreciable phosphorus. Significantly cheaper though a little worse is olive oil, about one gram per 80
ml, also an option is chloroform, ~1 g / 40 ml. Chemical desctruction of phosphorus in an apparatus is always an option, but as my experience shows
it can become coated in... whatever.... which can prevent further oxidation and leave with with a fire hazard later on. Someone should try the
reaction between aqueous copper sulfate and white phosphorus for destruction if the situation presents itself, it is one of the methods mentioned in a
book I read on the destruction of dangerous reagents in the labratory.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Just a useful detail, Ca3(PO4)2 can be converted to Ca(H2PO4)2 by reacting with HCl, and the dihydrogen phosphate should be much easier to reduce
(even if it turns to Ca(PO3)2!), while calcium triphosphate is cheaper than straight sodium triphosphate.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Are you sure? Sulfuric acid would do that to tricalcium phosphate, but HCl is too volatile and not as strong an acid as phosphoric acid.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
HCl is a strong acid, whereas phosphoric acid is considered a weak acid, especially after loosing two protons and forming
HPO<sub>4</sub><sup>2-</sup>. This means that the equilibrium here is shifted strongly to the
HPO<sub>4</sub><sup>2-</sup> side.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Hmm, not as strong as which hydrogen of phosphoric acid? 
I suspect this would work. Use of sulphuric produces the problem of separating the sulphate unless you go all the way to phosphoric acid.
Ideally I have the feeling there is a 'magic salt' mixture consisting of a low melting eutectic of metaphosphates that could be regenerated
over again with phosphoric acid.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
I just read over the lead phosphate reduction patent and it seems though there might be a way to do phosphorus reduction with controlled temps under
500C (sort of).
Let me explain for those that haven't read some of the previous posts or the patent.
According to the patent, lead phosphate or Pb3(PO4)2 is reduced under hydrogen or methane (natural gas comes to mind) with hydrogen resulting in the
highest yields and methane about 50% of that.
The reaction consists of three stages:
1. The Pb3(PO4)2 is heated up to 300C to drive off any existing water.
2. Once the temp hits 300C the hydrogen is turned on and the tempurature slowly raised to 500C. The hydrogen reduces the Pb3(PO4)2 by ripping off the
oxygen molecules and forming Pb3P2, aka lead phosphide.
3. Upon the cessation of evolution of water, the furnace is again slowly raised up to somewhere between 650-800C. According to the patent, small
amounts of PH3 are liberated at around 600C. This makes sense, the Pb3P2 probably starts to break down somewhere around 600C and thus liberates PH3,
which subsequently start to be reduced to H2 and elemental P at around 650C, so basically at the beginning of the reduction temp the phosphine being
liberated is not hot enough to break down.
What if one were to use small vessels for batch reduction of Pb3(PO4)2. They could be heated in a pot full of molten lead at the proper tempurature
more easily that way (everyone knows that without some sort of flux lead doesn't stick to metal worth a damn). The resulting Pb3P2 could then be
stored in a container with a dessicant. Once a sufficiant quantity has been made, some sort of reactant could then be slowly dripped thru the product
creating phosphine gas, which is routed to a glowing steel tube in fire, where it is effecively reduced to its elements, H and P.
This would eliminate the need for larger (read expensive) reduction vessels, easier cleanup, and less cost.
Alternatively, it would be much easier to react the aluminum with phosphoric acid, and reduce it in the same manner, however no literature that I know
of details this.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
another trivial experiment
The last few times I'd attempted some sort of phosphorus production, I started out with mixtures of phosphates and reducing agents. I've
been working in borosilicate test tubes, which aren't the most heat-resistant vessels, so I usually ended up melting the glass before I saw any
sort of reaction. Today was different.
2 g of lead wire were placed in a borosilicate test tube along with 1 ml of 85% H3PO4. This was heated in a propane torch flame, carefully at first as
water was driven off. Heating was increased, and the lead melted under the acid. After a couple minutes a thin stream of whitish smoke started wisping
from the test tube. The smoke had the characteristic smell of burning phosphorus. It occurred to me after a bit to turn off the light, and I saw a
mysterious and beautiful site: there was a greenish light appearing about halfway down the test tube. The light moved up and down the tube as the
heating was increased and decreased in intensity, probably representing the rate of production of flammable vapor vs. its interaction with the
atmosphere. After admiring the green glow for a few minutes, I broke off the experiment.
There was something else that appeared in the tube: a reddish coating on the glass above the area with the lead and acid. It looks like read led, so I
believe that some of the lead had volatilized and oxidized in the tube.
I found this experiment interesting because the phosphorus production took place entirely at or below a bright red heat, below the melting point of
borosilicate glass, while previous experiments rendered the glass unusably soft before showing any signs of success. I realize that test-tube
experiments of this sort will never lead to useful production but I find them interesting anyway.
Edit: after I let the tube cool and I broke open the end with the solidified acid and lead residue, I smelled a peculiar phosphorus odor. With the
lights out, I could see a faint glow on one particular chunk of residue, but there can't have been much of anything there since warming it in the
light didn't reveal any smoke or other visual signs of phosphorus.
[Edited on 5-11-2005 by Polverone]
PGP Key and corresponding e-mail address
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
evil_lurker, why not try heating the Pb3P2 formed with sulfur as was described by BromicAcid in the case of iron phosphide. Might give some results.
Besides the reaction temperatures (excluding the last step of phosphine liberation which I am excluding) are surely within reach. If the container was
sealed tightly enough a stream of dry hydrogen could be passed quite easily. I'd like to try using metal piping as in the case of Bromic, which
seems to be quite leak proof. Reducing with hydrogen does look interesting. Btw, nice results Polverone, the more I hear about this faint glow the
more I wish for the summer holidays to start 
Theory guides, experiment decides.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Actually I plan on going down to the steel yard sometime soon and pick up some decent diameter pipe, say in the 4-6 inch neighborhood and some sheet.
I can cut the pipe to the desired length, and simply weld on a cap on each end, and a iron pipe fitting on top for a crucible.
I do need to make some modifications to my "hellfire barrel" though. The last experiment in melting down an aluminum engine block was a
success, using a 55 gallon drum as a furnace. The bottom of the drum had a 1-2 inch hole burnt in it for the molten aluminum to run out, the side had
another hole burnt in it about 3 inches in diameter. In the 3 inch hole, a section of 2 inch copper pipe about 12 feet long was placed and hooked up
to a shop vac to provide forced air. The bottom of the barrel was loded with dry oak wood in fairly decent sized chunks. The engine block was set upon
this with the barrel top off, and lit with gasoline and a firecracker making a nice mushroom fire/ball cloud go up with a large "WHUMP". It
took about an hour, but I melted some odd 70 pounds or so of aluminum leaving all sorts of steel engine parts in the barrel along with some
uncombusted charcoal.
I know the inside had to hit at least 660 degrees to melt the aluminum, and probably hit the 800 required for the sodium hexametaphosphate, silicon
dioxide, and aluminum reduction.
I did not have a lid on the barrel, so I lost quite a bit of heat coming out the top. I'd say the flames were approx 6 feet high. It would
probably work better also if the air stream entering the barrel was at an angle rather than straight in.
One idea for a crucible that was considered was a small portable air tank. They are about $20, but the down side is they are a one use deal.
Another idea was to use several excesses of regular silica sand in a small crucible that has had the powdered sodium hexametaphosphate blended in and
using natural gas (mostly CH4, methane) as a reductant to reduce the P205 formed into P4. The excess silica sand would create a P205 coated gas
permeable "matrix" thus allowing better yields since the P4 vapor could more easily leave the crucible.
Experiment will show I suppose.
|
|
|
ChemicalBlackArts
Harmless

Posts: 8
Registered: 17-8-2005
Location: Michigan
Member Is Offline
Mood: No Mood
|
|
Pshhh, why make any allotrope of elemental phosphorus? Phosphorus is much better put to use in VX! C11H26NO2PS
--Chemistry in my veins--
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Because white phosphorus looks so damn cool and toxic. 
Also makes for a good firestarter.
sparky (~_~)
P.S. I'd only play with VX if I had 10 vials each of pralidoxime and atropine on hand, thank you very much. 
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
praseodym
Hazard to Others
  
Posts: 137
Registered: 25-7-2005
Location: Schwarzschild Radius
Member Is Offline
Mood: crazy
|
|
Different allotropes of phosphorus could be used for different uses. For example, white phosphorus is used in military applications as incendiary
bombs, for smoke-screening as smoke pots and smoke bombs, and in tracer ammunition, while red phosphorus is essential for manufacturing matchbook
strikers, flares, and, most notoriously, methamphetamine.
Basically, phosphorus exists in three allotropic forms: white, red, and black. Other allotropic forms may exist. The most common are red and white
phosphorus, both of which consist of networks of tetrahedrally arranged groups of four phosphorus atoms. The tetrahedra of white phosphorus form
separate groups; the tetrahedra of red phosphorus are linked into chains. White phosphorus burns on contact with air and on exposure to heat or light.
Phosphorus also exists in kinetically and thermodynamically favored forms. They are separated by a transition temperature of -3.8 °C. One is known as
the "alpha" form, the other "beta". Red phosphorus is comparatively stable and sublimes at a vapor pressure of 1 atm at 170 °C
but burns from impact or frictional heating. A black phosphorus allotrope exists which has a structure similar to graphite – the atoms are arranged
in hexagonal sheet layers and will conduct electricity.
|
|
|
tramp
Harmless

Posts: 1
Registered: 25-8-2005
Member Is Offline
Mood: No Mood
|
|
white phosphorus dissolves readily in carbon disulfide...
a favorite game of mine in high school (this was several decades ago...) was to dissolve WP in CS2, then fling the liquid on a wall in the dark... it
was cool to watch the wall begin glowing green then errupt in flames...
|
|
|
budullewraagh
Hazard to Others
  
Posts: 168
Registered: 1-8-2004
Location: new york
Member Is Offline
Mood: Aliphatic
|
|
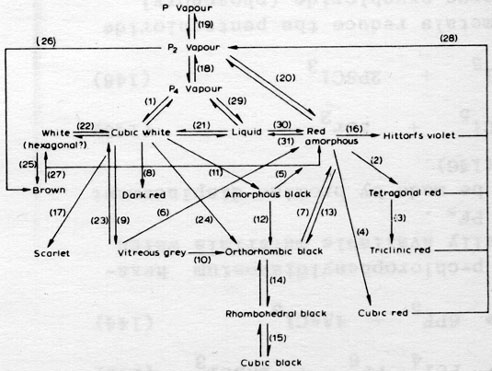
but there are many other allotropes of phosphorus. please see attachment.
i have heard of blue and orange phosphorus as well, although they, like yellow, may not be true allotropes.
a key to the chart:
1] High vapor pressure at room temperatures, [2] heat at 540 C,
[3] heat at 550 C,
[4] heat at 600 C,
[5] heat at 125 C,
[6] heat at 400 C,
[7] heat at 550 C,
[8] heat at 300 C at 8000 atm,
[9] heat at 380 C with Hg or above 250 C at 12 kb,
[10] heat at 400 C with Hg for days,
[11] heat at 200 C at 12000 atm,
[12] heat at 200 C at 15000 atm,
[13] heat at 200 C at 12000 atm,
[14] reversible trasition 50-100 kb,
[15] reversible transition 110 kb,
[16] recrystallize from molten Pb,
[17] heat a PBr3 solution,
[18] reversible transition at 900 C,
[19] reversible transition at 1700 C,
[20] reversible transition at low pressure,
[21] reversible transition at 44.1 C (but can supercool),
[22] reversible transition at -77C or +64 under 1200 atm,
[23] sublime under vacuum,
[24] heat at 220 C at 12 kb,
[25] irradiate with UV at -190 C,
[26] condensation of P2 vapor at -196 C,
[27] heat above -100 C,
[28] heat at low pressure,
[29] boils at 280 C,
[30] heat at 300 C or expose to light or X-rays,
[31] melt about 600 C
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Yeah, but this discussion should be in the thread on phosphorus allotropes, as this table is already there as is the discussion of the different
allotropes.
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
WP and KClO3 detonation
| Quote: | Originally posted by tramp
white phosphorus dissolves readily in carbon disulfide...
a favorite game of mine in high school (this was several decades ago...) was to dissolve WP in CS2, then fling the liquid on a wall in the dark... it
was cool to watch the wall begin glowing green then errupt in flames... |
If you drop a few drips of this solution (WP in CS2) on a small pile of Potasium chlorate (KClO3), wait a wile until a portion of the CS2 has
evaporated, a hugh detonation will take place.
CAUTION: This experiment is very dangerous, for a powerful detonation takes place, with a big bright flame.
How is this for high school fun ?
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
It spontaneously catches fire? I wonder where the activation energy comes from.
|
|
|
woelen
Super Administrator
        
Posts: 8082
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
White P is very reactive and on a warm day in summer it can catch fire without heating. I recently received some white phosphorus and I was quite
scared when I moved it from one container to another. As soon as I took it from the water a white smoke was released and I put it under water in the
other container as fast as I could.
The reason for this reactivity is the strain in the P4 molecules. It is like a spring, which is severely stressed and which is about to break. Only
little agitation is needed to break the spring.
BTW, red P and KClO3 also can ignite spontaneously. I put some KClO3 on the ground and added some red P, with the intent to mix them while on the
ground, but due to wind, the chems were scattered over a larger area. I took a little stick and wanted to scrape the chems on a little heap. As soon
as I touched the chems, a bright white flame was given, some molten KClO3 was sprayed around (also some on my hand  ) and all stuff was gone. Fortunately the amounts were small, not more than 100 mg total. I was really surprised,
because the chems were not even mixed, there was just a layer of red P on top of a thin layer of KClO3. ) and all stuff was gone. Fortunately the amounts were small, not more than 100 mg total. I was really surprised,
because the chems were not even mixed, there was just a layer of red P on top of a thin layer of KClO3.
[Edited on 3-9-2005 by woelen]
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
I must have missed that W, sorry.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
P + KClO3 is well known to be rather dangerously unstable, after all. 
Tim (has put a crystal of KClO3 between two match strike pads and struck with hammer)
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Yes, I'm well versed in the processes of natural selection. 
|
|
|
| Pages:
1
..
4
5
6
7
8
..
60 |