| Pages:
1
..
4
5
6
7 |
forgotpassword
Harmless

Posts: 47
Registered: 12-8-2014
Member Is Offline
Mood: No Mood
|
|
I don't mean to come across as offensive but I don't really understand what you are saying.
Paraffin is better of grease?
Are these two different compositions?
Is KClO3 and Paraffin better than Vaseline?
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
LL, the stated density of your Cheddite charge is extremely low. Normally for these types of chlorate and hydrocarbon Cheddites the density is much
closer to 1.3g/cc than it is to your stated value of 0.65g/cc.
forgotpassword, I doubt there would be much difference in explosive performance between when Paraffin and Vaseline are used. Paraffin wax and Vaseline
are both mixtures of heavy hydrocarbons that come off near the bottom when crude oil is fractionally distilled. I suppose there could be some physical
property differences that might be important, melting point, etc, but, chemically, as fuels they are very similar.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I just want to tell you all that Cheddites made only, with as fuel counterpart, paraffine, vaseline, wax or alcanic fuels are:
-not very powerful
-not very dense
-not very sensitive
--> @Forgotpassword
Especially if not wel confined (confinement by hand pressing, selfconfinement by 165g, and rubber balloon is highly not efficient confinement for such
insensitive mixes)
Best confinement is acheived in borehole or inside Iron-steel pipe 4mm thickness and 2-5cm diameter!
I have made Cheddites in my young years (20 years ago) for new year eves... with NaClO3 and KClO3 but the fuel counterpart was nitrobenzene,
dinitrobenzene, dinitrotoluenes oil, nitro and dinitronaphtalene waxes.
Those fuels are more interesting because they contain more carbon and less hydrogen, their density is higher, they contain some active oxygen what
allows a reduction of the amount of chlorate and inert resulting salt (NaCl or KCl); and last but not least those fuel are active explosive in the
case of dinitrocompounds...
They display VOD in the range 5-7 km/s and are much more sensitive to initiation...but they stil need strong confinement for correct detonation. The
NaClO3 and KClO3 are sometimes porous prilled like NH4NO3 for fuel impregnation.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
forgotpassword
Harmless

Posts: 47
Registered: 12-8-2014
Member Is Offline
Mood: No Mood
|
|
I will do another test when my chlorate cell makes enough.
I will use your improvements. I will put the Vaseline and Chlorate in a blender to thoroughly mix the two components.
I will not however put cheddite in an iron pipe as I am not looking to make a pipe bomb, I am not sure what you mean by a borehole, but the iron pipe
idea seems too dangerous.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
cheddite
Henning Brand says: I suppose there Could be some physical property Differences That Might Be Important, melting point, etc, but, Chemically, fuels
and They Are Very Similar.
That is true. Vaseline evaporate faster. Into detonation. A cool burning. It is wrong. But, this is only a small difference. My
recommendation is based on limited options Forgotpass. You need to use good quality fuel. As PHILOU writes and designs. Top find or produce ammonium
nitrate. Long mixing Vaseline will not help. Use a blender to mix KClO3 and fuel? Carefully.
LL
|
|
|
forgotpassword
Harmless

Posts: 47
Registered: 12-8-2014
Member Is Offline
Mood: No Mood
|
|
What about Kerosene and KClO3? That may be worth a try as can be seen here:
<iframe sandbox width="420" height="315" src="//www.youtube.com/embed/NQnyFisrAbk" frameborder="0" allowfullscreen></iframe>
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
| Quote: |
What about Kerosene and KClO3? That may be worth a try as can be seen here:
|
"Paraffin" or "paraffin oil" are British/Aussie/colonial terms for what is called Kerosene in the USA.
Thanks for link to video- I am VERY pleased to see an amateur posting on VOD measurement circuitry!
[Edited on 5-11-2014 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
full detonation
Perfectly processed video. Quality work. Full detonation. It is seen detonation wave. Is it possible? Thank you for this video. Forgotpassword learns
quickly! A good deformation of the steel plate. Great praise before long burning fuse....
LL
Measuring the velocity of detonation is simple and ingenious. I have a lot to learn. Hats off.
[Edited on 5-11-2014 by Laboratory of Liptakov]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Great video!
I think at least in the North American texts that paraffin refers to paraffin wax in Cheddite compositions. Heavy petroleum oils are also used in some
compositions of course though.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
forgotpassword
Harmless

Posts: 47
Registered: 12-8-2014
Member Is Offline
Mood: No Mood
|
|
I'm really sorry to say that I didn't make that video, it was just an example of Kerosene and KClO3.
Sorry to disappoint you, LL and Bert.
However, it does give good information on the VOD of Cheddite.
[Edited on 5-11-2014 by forgotpassword]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
measuring
Nothing happens Apology accepted, this video is very informative. Measuring assembly is very simple. This is important. Just what I was looking for a
long time. Digital oscilloscope and ordinary (cheap) lead wires. No optical fiber. A simple converter. Another important thing: from the curve on the
screen is easy to read, where exactly subtract values. And even in the case where the curve will be unclear, imprecise weak (weak ionization).
Yet, thank you. .....
Another thing: Intermolecular mixture (wet way) KClO3 84 + Hexamine 16 + Al flakes 2, gives about 20% higher brisance. Just tested, photo later.
LL
|
|
|
forgotpassword
Harmless

Posts: 47
Registered: 12-8-2014
Member Is Offline
Mood: No Mood
|
|
Thank you for testing for us!
I do not have access to Hexamine or Aluminium Powder.
I will test KClO3 + Kerosene later.
What did your initiator consist of?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
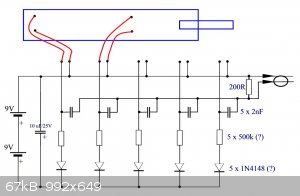
scheme
I use 1 g ETN / 0.2 LA. Here is a diagram of the video. Some values are not readable. They are supplemented with estimates. 360 is a low
resolution.
LL
|
|
|
forgotpassword
Harmless

Posts: 47
Registered: 12-8-2014
Member Is Offline
Mood: No Mood
|
|
I thought you said my 3g was not sufficient to set off my cheddite?
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
In U.S. patent #3155554, "Liquid Blanketed Chlorate Blasting Agent", Melvin Cook et al. suggest that sodium chlorate based explosives are more
powerful than those using potassium chlorate. An example in the patent is 88% NaClO3 & 12% fuel oil.
Attachment: US3155554A.pdf (183kB)
This file has been downloaded 669 times
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Sodium chlorate does make slightly more powerful explosives than potassium chlorate. Sodium atoms are much lighter than potassium atoms, so a pound of
sodium chlorate contains more oxygen than a pound of potassium chlorate. However, sodium chlorate is also very hygroscopic and potassium chlorate is
not. Sodium chlorate also requires much less energy to initiate its decomposition, so it generally makes much more sensitive explosives than potassium
chlorate (which are already very sensitive).
Quote: Originally posted by Bert  |
If you can't interpret the melt points and enthalpy of formation of the various common oxidizers as signposts to the relative sensitivities of the
mixtures made with them, you need to learn and keep in mind physics as it relates to explosives chemistry.
As for the melting point:
Solid with solid reactions proceed only at the contact points between particles, limiting the surface area available for reaction relative to that of
a solid immersed in a liquid or gaseous reactant. To realy get things going at least one the reactants has to become a liquid or gas, allowing it to
contact the COMPLETE surface area of other reactants - Hence the influence of melting point on sensitivity. Most pyrotechnic reactions really start to
move around the temperature that one of the ingredients melts, generally the oxidizer. A low melting fuel like Sulfer or lactose can help provide a
similar effect. How is a mixture of Sodium chlorate and Sulfer or lactose likely to behave? (Hint: use very small quantities if you want to see).
Look up the common oxidizers melt and decomposition temperatures, list them in order from low to high- Notice Sodium Chlorate has a rather low melting
point, so it's going to take less energy to get the party started there.
About enthalpies of formation:
If energy was given off in the formation of a compound, you will need to provide at least that amount of energy to reverse that process-
Look at oxidizing Aluminum to Al2O3, which has a (relatively high!) enthalpy of formation of -1675.7 kJ*Mol. Lots of heat was given off there! (it
also has a VERY high melting point...). Do you use it as an oxidizer???
Look at what I listed above for the chlorates:
Sodium Chlorate melts @ 248 C., enthalpy of formation -53 kJ*Mol
Potassium Chlorate melts @ 356 C., enthalpy of formation -391kJ*Mol
Do you use THEM for oxidizers? From the difference between those figures, which is going to make a more sensitive mixture, all other things being
equal?
Further reading:
Conkling: Chemistry of Pyrotechnics- basic principles and theory
|
[Edited on 6-11-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
E300
Tested composition: KClO3-70 + E300 (vitamin C)-30.2 + Fe2O3-1. Very good power. VoD about 3200m/s. 2 mm sheet torn. Charge 9g, hand presse. Prepare
wet way. This mixture would require additional tests.
LL
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
There are a number of perchlorate based explosive patents that similarly rely on intimate mixing through dissolving fuels, oxidizer or BOTH, some of
which do not even require the mixtures be dried-
This thread has long intrigued me.
Gelled Perchlorate Explosives
[Edited on 7-11-2014 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Bert you might find the attached patent interesting ("Liquid and Slurry Explosives of Controlled High Sensitivity").
One of the examples is a perchlorate-based liquid explosive (which has lots of water), which detonates at 2300 m/s, and the addition of 10% of Al
powder brings it up to 5300 m/s.
Attachment: US3765967.pdf (405kB)
This file has been downloaded 763 times
|
|
|
Etanol
Hazard to Others
  
Posts: 188
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | Tested composition: KClO3-70 + E300 (vitamin C)-30.2 + Fe2O3-1. Very good power. VoD about 3200m/s. 2 mm sheet torn. Charge 9g, hand presse. Prepare
wet way. This mixture would require additional tests.
LL |
Hello! I am glad that someone is interested in measuring the VoD.
It is really value you measured? Cool!
Do not expect from such mixtures.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
VoD cheddite
It's not an exact value. These compositions will require further testing. Perhaps it is only 2,900 m / s or even 3500 m / s. If you do spherical
charge such as 200g, VoD will be higher, of course. I do not know. It's just a rough estimate. I'm not a fan KClO3 + fuel. Further tests will not be
repeated. Someone else can try.
LL
[Edited on 11-11-2014 by Laboratory of Liptakov]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
WARNING
Warning. A mixture of KClO3 + E300 can explode at any moment. Weighed only 20 grams. Do not mix, not to try.
LL
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Did you experience an accidental explosion with the chlorate/ascorbic acid mixture?
Ascorbic acid is a fairly weak acid, but perhaps it is strong enough to generate ClO2 from chlorate in the presence of moisture, leading to
spontaneous combustion/explosion?!
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
green ball
Yes, that's exactly what happens. The mixture was made wet. Nose smelled of chlorine and hydrogen chloride. The mixture became green. Established
plastic. 20 g green balls.
After 5 minutes, erupted. VoD about 300 m / s. As gunpowder. The sphere was cold. There was no visible smoke. Just burst without warning. No damage
was, no injuries.
LL
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
More excitement than I would want... Spare clean underwear should NOT be a necessary supply in a well run explosives testing lab!
As convenient as cheddite and the several variations on that family of mixtures may seem to those amateurs running a chlorate cell, chlorate based
high explosives have been long abandoned for commercial/military use...
Lower performance, higher sensitivity, chemical reactivity issues. What's not to like!
From Hudson Maxim- Dynamite Stories
| Quote: |
SCATTERED
I was once called as an expert to visit a dynamite plant where a new kind of high explosive was being manufactured instead of the ordinary
nitroglycerin dynamite. It consisted of a mixture of chlorate of potash, sulphur, charcoal and paraffin wax. Its inventor had given it the reassuring
name of Double X Safety Dynamite. A quarry-man in a nearby town had, with his safety-ignoring habitude, attempted to load a hole with the stuff, using
a crowbar as a rammer, with the result that he set off the charge, and the crowbar went through his head. This unscheduled eventuation aroused the
apprehension of the president of the company, who was also its backer. He began to grow suspicious about the safety of the material. Being so much
interested, he went with me on my visit of inspection. We left the train at a siding about a mile from the works, and had just started in their[137]
direction when there came a sudden boom and roar, and the earth shook. Over the powder works there rose a huge column of black smoke, flaring wide
into the sky. We found a great crater where the mixing house had stood. Three men were working in the building when the explosion occurred. A
fortunate survivor who had left the place a moment before to go for a bucket of drinking water, was walking about the crater, apparently searching for
something among the scattered remnants. As we approached him, he sadly said: “I can’t find much of the boys. I guess you’ll have to plow the
ground if you want to bury them.”
|
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
| Pages:
1
..
4
5
6
7 |