| Pages:
1
..
4
5
6
7
8
..
27 |
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
This is going to be a multi-post. I used scifinder to look up pentazoles. So attached is some toilet reading
A few papers.
[Edited on 9-8-2007 by The_Davster]
Attachment: pentazole.pdf (110kB)
This file has been downloaded 1894 times
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
2
Attachment: pentazole2.pdf (137kB)
This file has been downloaded 2795 times
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
3
Attachment: pentazole3.pdf (456kB)
This file has been downloaded 3200 times
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
One question micht be relevant in all this, if a phenyl-ring adds stability to the pentazolyl-group, why remove it? How good would
Ph-N<sub>5</sub> be as a ligand? This appears not to have been researched...
Also, it seems like a molecule such as antracene might be able to stabilize it even better.
Thanks for the papers btw, very nice.
[Edited on 10-8-2007 by Nerro]
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
ssdd
Hazard to Others
  
Posts: 211
Registered: 13-4-2007
Location: Central Canada
Member Is Offline
Mood: Hypergolic
|
|
OK so I was going to attempt to make some Nickel Hydrazine Nitrate.
But before I do so I am trying to gather as much info as possible, and I was wondering if anyone knows the structure of NHN.
Or better yet, if anyone has a program that can generate these structures.
Any info would be great.
-ssdd
All that glitters may not be gold, but at least it contains free electrons.
-- John Desmond Baernal
http://deepnorth.info/ |
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Get most any version of ChemOffice. You can have hours of fun with it and it also has 3D modeling effects, etc, etc. One thing that I like to do is
import the structure of items I found in the Merck Index (v13+) program (it allows for this specifically) and model what I am working with. then you
can add to it from what your project is at the time. You save the basic structure for later, etc. - It's a lot of fun. You DON'T have to get the very
latest one. I did and it's like getting the latest MS product.....bell & whistles. Most recent versions are just fine.
|
|
|
Ballermatz
Harmless

Posts: 19
Registered: 17-7-2007
Member Is Offline
Mood: No Mood
|
|
[Edited on 24-12-2007 by Ballermatz]
|
|
|
Ballermatz
Harmless

Posts: 19
Registered: 17-7-2007
Member Is Offline
Mood: No Mood
|
|
[Edited on 24-12-2007 by Ballermatz]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
| Quote: | | woelen has described this compound in detail on his homepage already, including the fact that it explodes upon heating - however one important detail
that has not been mentioned so far is that it explodes violently upon impact too! I'd say it is even more sensitive than TACP. Since there is nothing
in this complex to be oxidized, the explosion must be caused by a sudden loss of its oxygene. Thus mixing with anything combustible will certainly
increase its explosive powder. |
I have 15 grams or so of this compound around for approximately 18 years already  . Do you think it is wise to destroy it and not keep it around any longer? I did not know it is impact sensitive too. I'll do some tests myself and
see how sensitive it actually is. After all those years of storage, the compound still is very energetic, so this at least is very stable, when stored
in a dry and air-tight container. . Do you think it is wise to destroy it and not keep it around any longer? I did not know it is impact sensitive too. I'll do some tests myself and
see how sensitive it actually is. After all those years of storage, the compound still is very energetic, so this at least is very stable, when stored
in a dry and air-tight container.
[Edited on 22-10-07 by woelen]
|
|
|
Ballermatz
Harmless

Posts: 19
Registered: 17-7-2007
Member Is Offline
Mood: No Mood
|
|
[Edited on 24-12-2007 by Ballermatz]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Nah, it could just be unusually sensitive to thermal anisotropy and vacuum fluctuation. 
Tim
|
|
|
Nixie
Hazard to Others
  
Posts: 490
Registered: 12-12-2006
Member Is Offline
Mood: ?
|
|
| Quote: | Originally posted by Ballermatz
50mg give such a loud report that it is paineful in the ears. |
Man, I hope you realize that permanent, cumulative hearing damage occurs from levels even below the threshold of pain.
\"Good is a product of the ethical and spiritual artistry of individuals; it cannot be mass-produced.\" --Aldous Huxley
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Well, except it's going to be too unstable probably ... and OB wise...is there any? 
Very nice work above ... do you have pictures of the above compounds?
With your resources, perhaps it's worth testing other transition metals, such as Ni, Cd, Cu etc?
For instance, copper hydrazine, guanidine, imidazole, pyridine, dinitrophenylhydrazine perchlorate, and the nitrates etc etc
We need a comprehensive review of all these things! 
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Ballermatz
Harmless

Posts: 19
Registered: 17-7-2007
Member Is Offline
Mood: No Mood
|
|
[Edited on 24-12-2007 by Ballermatz]
|
|
|
Ballermatz
Harmless

Posts: 19
Registered: 17-7-2007
Member Is Offline
Mood: No Mood
|
|
Merry christmas to all!
[Edited on 24-12-2007 by Ballermatz]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Of course I admire the photos- But feel some misgivings about storing energetics known or suspected of being primary explosives in glass!
|
|
|
jlithen
Harmless

Posts: 6
Registered: 2-12-2004
Member Is Offline
Mood: I feel great!
|
|
Hi!
I must comment the sensitivity of NHN.
I have prepared it many times in about 10-20g batches and it is extremely difficult to filter. After drying and at some point grinding it (do this in
small ammounts if it is already dry) it is a very flammable powder that does not seem to detonate easily unless confined.
I have tested friction sensitivity by means of a mortal and pestle. It is very well possible to make it detonate. It is deffinitely much more
sensitive than TNT that I think someone compared it with. Anyway I have found it to me much less sensitive than e.g. PbN3. Maybe in the order of ETN
or MHN.
I have also stored it for about 2 years at some point and haven't noticed any change. When i tried to make the same salt of Cobalt it was a bit weaker
but needed less confinement to detonate in 10g quantities. Anyway it degraded in 2 weeks to something useless (water + something I guess) When burned
the H from NH3 and O from -NO3 at least forms H2O, the metal forms an oxide and N2 is also formed.
Sorry for al typos et.c. I'm really in a hurry right now
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Somebody asked for structure of NHN, it is attched below. Made in ChemDraw.
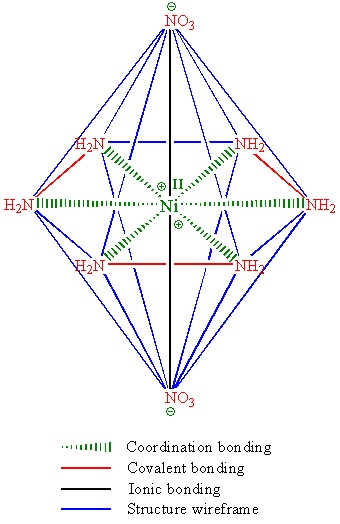
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Ni<sup>2+</sup> only needs ten more electrons to fill up all of it's orbitals. You have too many ligands.
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
It's flat planar? That doesn't make sense, shouldn't it be an octahedral coordinate like trisoxalatoferrate (and for that matter, Ni(en)3 and more)?
Tim
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by Nerro
Ni<sup>2+</sup> only needs ten more electrons to fill up all of it's orbitals. You have too many ligands. |
Bingo! You just got point, why it is unstable and explosive.
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by 12AX7
It's flat planar? That doesn't make sense, shouldn't it be an octahedral coordinate like trisoxalatoferrate (and for that matter, Ni(en)3 and more)?
Tim |
It should be, but my reference in russian chemistry book points at this structure. However all this subject is doubtfull, correct answer may be
acchived only by dirrect physical measurements.
[Edited on 22-2-2008 by Engager]
|
|
|
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
Tetraamine zinc peroxide. [Zn(NH3)4]O2 I searched but couldn't find any information about it. Does anyone know its properties or how to make it?
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
What makes you think Zn(NH3)4O2 even exists? ZnO2 is covalently bound unlike the Zn salts.
|
|
|
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Axt
What makes you think Zn(NH3)4O2 even exists?
|
I didn't know exactly if it exists, I only heard once about it and saw the formula.
|
|
|
| Pages:
1
..
4
5
6
7
8
..
27 |