| Pages:
1
..
54
55
56
57
58
..
60 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I like your design, but would make a few changes:
1. Change carbon to aluminum.
2. Monitor all condensers for temperature and control to the optimums.
3. Incorporate cyclone separators where needed to separate gasses from the liquid Phosphorus.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
metalresearcher
National Hazard
   
Posts: 757
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
This would be a rather compled design. It needs indeed strict temperature control in the condenser. But what is actually the problem that CO and P4
arrive together in the condenser? They don't react with each other and can easily be separated by condensing the P4, as CO remains a gas.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
My suggestions are for producing clean P at high yield, not for convenience of the operator.
Use of carbon vs aluminum will greatly increase gas formation, and decrease the necessary retort temperature. My experiences with aluminum and SiO2
produced a nasty voluminous slag that contaminates the P.
Use of a centrifuge for gas-liquid separation would be wonderful but indeed too complicated. A cyclone might work just as well and be far simpler.
[Edited on 12-10-2017 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
@metalresearcher i think the problem is that the combined pressure of the gasses being produced and the high temp make condensing the P4 a quite
challenging, a liebig type condenser may not be enough to condense all the P4 and a large amount may possibly be lost out the top (hence my idea of
using a stopchock).
Unfortunately I have no idea just how quickly the reaction proceeds beyond what BluePlanet1 described, what he described seems quite intense.
A possible simple solution, if the condenser is not enough would be to pack the condenser with a material with high surface area such as glass wool.
@Magpie i do like the cyclone idea but im a tad confused as to its operation.
Is the spinner supposed to rotate solely by the pressure of the gases being pushed through or would it have to be electrically driven.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
A cyclone has no moving parts. It spins the gas into a vortex. Denser material is flung out to the edge and funnels to the bottom. Light gas leaves
at the centre top so that gas flow is maintained.

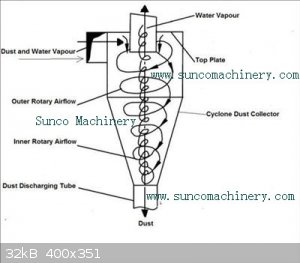
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
The cyclone separator would appear to be a lot easier to engineer than i had originally thought.
One problem though: from what ive read on them cyclone separators are only capable of separating particulates or liquid droplets from a gas flow, they
cannot separate 2 gasses at all.
This means that we would still need to condense the P4.
A water jacket around the cyclone housing would be the minimum required to accomplish this. Achievable but it makes other methods look more appealing.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I'm really confused as to why the "just use a longer pipe and submerge it very deep in water" method is not being taken more seriously. Using water eg
one meter deep should condense most of the phosphorus. It's not like you have any other condensates to worry about.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
First the P4 must be condensed using a proper condenser. Then it will drop out the bottom of the cyclone into a water filled receiver. Gasses will
leave the top port of the cyclone, hopefully relatively free from P4. Cyclone design will be important, as well as condenser design and temperature.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | | First the P4 must be condensed using a proper condenser. Then it will drop out the bottom of the cyclone into a water filled receiver. Gasses will
leave the top port of the cyclone, hopefully relatively free from P4. Cyclone design will be important, as well as condenser design and temperature.
|
So is the problem believed to be that the phosphorus condenses to a fine mist or dust that does not easily settle?
Looking at the short exit pipes and large bubbles in the water I would think cooling is the main problem. One end of the exit pipe is at +1000C with
the gaseous CO and P even higher and only about 200mm of air cooled pipe to get the temperature down to say 200C.
The large bubbles so near the surface probably don’t wash out the P or cool it very efficiently particularly given the flow of CO.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, condenser design is important and less than optimum design was the cause of many of my troubles. Even though I generally used aluminum as
reductant the gas/P/slag rush could be explosive. I used an air condenser made of 1/2" electrical conduit (EMT).
Gruson did excellent work with the help of his science teacher and some welding craftsmen. Here is a link to his work: https://www.sciencemadness.org/whisper/viewthread.php?tid=65...
Unfortunately his photos are no longer available.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
e14
Harmless

Posts: 7
Registered: 23-10-2016
Member Is Offline
Mood: No Mood
|
|
Shouldn't this thread be erased? The DEA lists it. So, how easy is this stuff easy to make?
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Compared to the risk of the experiment I would not worry about the DEA, unless you plan to go into production.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
But don't do it on your front lawn or poison your neighbours .
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
| Quote: |
Shouldn't this thread be erased? The DEA lists it. So, how easy is this stuff easy to make?
|
 The DEA can go fuck themselves, phosphorous is an element and a fundamental
one for life, why the hell would we restrict information because a bunch of degenrate apes want to misuse phosphorous for illegal or immoral purposes. The DEA can go fuck themselves, phosphorous is an element and a fundamental
one for life, why the hell would we restrict information because a bunch of degenrate apes want to misuse phosphorous for illegal or immoral purposes.
That aside, the items on that list are not illegal, we are technically allowed to make them, however the DEA or other government
agencies from what i understand do also have the right to lay charges on us if we are caught with possession of those materials.
If you spend some more time lurking the older threads you will likely come across several threads discussing the preparation of scheduled compounds
such as lysergic acid and anthranilic acid.
The forum cares little for the bickering stupidity of mortals.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
e14 is one of a number of low post, dormant accounts that reactivated recently, posting gibberish or, if intelligible, quite different from their
earlier activity.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
derped. sorry
[Edited on 2-3-2018 by Rogeryermaw]
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
mmmm phosphorus! deelish! man...this thread has moved quite a bit since i've been away.
Quote: Originally posted by clearly_not_atara  | | I'm really confused as to why the "just use a longer pipe and submerge it very deep in water" method is not being taken more seriously. Using water eg
one meter deep should condense most of the phosphorus. It's not like you have any other condensates to worry about. |
it doesn't take much fluctuation in temperature to cause a suction. i would be afraid of a steam explosion blasting hot slag and gaseous phosphorus
all over.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Easy to make? Hard to make?
Seemingly impossible for some of us to make Phosphorus, but not very difficult for Rogeryermaw.
Has a nice series of Youtube videos. https://www.youtube.com/watch?v=mibM4WUx74Q
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
I haven’t seen anyone try this in this thread yet so I thought I’d give it a go.
I just did a little experiment with P2O5 and some dark Al powder. I wanted to see it the Al reduced the oxide and it seems to. But very quickly. So
what I did was mix small amounts of Dark Al powder and P2O5 maybe 100-200 mg of each but a little more oxide than metal. not stochiometric amounts
though. I did not measure them as I wanted to see what happened just as a quick test. The powders were mixed quickly before moisture affected the
oxide too much and put the mixture into a 50cm quartz tube with a plug in one end and proceeded to heat the mixture with a MAPP torch from below.
After about 30 seconds there was a wispy white smoke coming from the pile and a moment later there was a quite energetic reaction and a release of gas
which kind of acted like a cannon. The whole tube was coated in what I assume to be red phosphorus and it was instantaneous.
The observation of the tube after was interesting. There was a bit of smoke in the tube and some white phosphorus residue which was smoking. The tube
had the unmistakable odour of phosphorus. It appears that the reaction is way to fast to be of any use to make white phosphorus this way. I may try
again with some 400mesh bright Al and see if it slows any and maybe reduce the heat a bit. I don’t have Time at the moment but maybe tomorrow.
Here’s a couple of pictures.
  
[Edited on 14-6-2018 by NeonPulse]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Maybe add a heat sink like CaF2, or some other reaction-slowing mechanism? I seem to remember it working for blogfast25's attempts at manganese
thermite, where the manganese metal would boil off - and this seems quite similar in general principle.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Looking at the short exit pipes and large bubbles in the water I would think cooling is the main problem. One end of the exit pipe is at +1000C with
the gaseous CO and P even higher and only about 200mm of air cooled pipe to get the temperature down to say 200C.
The large bubbles so near the surface probably don’t wash out the P or cool it very efficiently particularly given the flow of CO.
From the videos I've seen on YouTube one guy has a gal steel pipe coming out of his furnace 2 foot in length total going into the bottom of a 250 ml
erlenmeyer which is open to the atmosphere and the flask let's off flames that snap and crackle with white smoke which is probably p4 vapors
igniting.if he used a longer steel pipe and a CHILLED bubbler system ie: 4 much larger flasks or bottles he would probably quadruple his yield.he
only got like one or two grams a run.I reckon most of it burned off.
[Edited on 13-8-2018 by draculic acid69]
|
|
|
JScott
Hazard to Self
 
Posts: 51
Registered: 23-8-2018
Member Is Offline
|
|
Getting ready
You may have seen that subject line, how the heck do you remove accidental postings?
Anyhow, after seeing NeoPulse's 'proof of concept' video I wanted to recreate that. I don't have access to a tank of Argon, but I can easily get a
small can of Ar intended for wine preservation. About 15$ on Amazon/Ebay.
I made this small balloon 'equalizer' to make use of this gas. A small hose clamp to bleed off a small flow of Argon. I wouldn't think it would be
much, just what is needed to be certain all oxygen has been removed from the tube, correct?
So I bent a few tubes and I'm waiting for enough time to get it done. Here's the 'apparatus'. Will there be enough gas in this balloon to get through
the procedure? I should probably consider another hose to top off the balloon mid way should I run low.
Anyone else familiar with this video? Have any suggestions?

|
|
|
Hunterman2244
Hazard to Others
  
Posts: 105
Registered: 6-6-2018
Member Is Offline
|
|
I think refluxing a mixture of magnesium, SiO2, charcoal, and phosphoric acid, should yield white phosphorous. Maybe a metal paint can on a hot plate,
a metal pipe coming off the top cooled by a fan, and some tubing leading into water. If you use clear tubing, either sunlight or a uv light could
convert it to red. Maybe instead a tube, a one way valve off the top of the pipe that leads into a container of water to stop the P from getting stuck
in the tubing. Like one of those things for making beer that lets the CO2 out, but keeps it sealed. Epoxy it onto a hole in the bottom of a tall
contained so it bubbles through as its own condenser.
|
|
|
JScott
Hazard to Self
 
Posts: 51
Registered: 23-8-2018
Member Is Offline
|
|
@Hunterman2244,
Thanks, when I scale up, it is my intention to work in a metal apparatus. However, I am a ways from there at this point. As mentioned, I really just
wanted to recreate what I had seen in that video. Though it only appeared to be partially successful, I thought for me, it would make a good first
step.
Partial successes can be instructional, and a bit more fun than absolute failure ;-) Further, I have been stymied by not having any inert gases on
hand. I was sent down this path when I found that wine connoisseurs used Ar to replace the air in opened bottles. Though I'm sure 15$ for that much
gas is a gouging, it's a lot cheaper than buying even the smallest refillable canister of Argon.
Any experience I can gain using these small, easily located cans of Argon will be helpful until I can afford more substantial supplies.
Still, your advise is right on point and very much inline with what I hope to do as I move further along in my studies. Thank you very much for
getting back to me.
[Edited on 9-1-2018 by JScott]
|
|
|
Hunterman2244
Hazard to Others
  
Posts: 105
Registered: 6-6-2018
Member Is Offline
|
|
Quote: Originally posted by JScott  | @Hunterman2244,
Thanks, when I scale up, it is my intention to work in a metal apparatus. However, I am a ways from there at this point. As mentioned, I really just
wanted to recreate what I had seen in that video. Though it only appeared to be partially successful, I thought for me, it would make a good first
step.
Partial successes can be instructional, and a bit more fun than absolute failure ;-) Further, I have been stymied by not having any inert gases on
hand. I was sent down this path when I found that wine connoisseurs used Ar to replace the air in opened bottles. Though I'm sure 15$ for that much
gas is a gouging, it's a lot cheaper than buying even the smallest refillable canister of Argon.
Any experience I can gain using these small, easily located cans of Argon will be helpful until I can afford more substantial supplies.
Still, your advise is right on point and very much inline with what I hope to do as I move further along in my studies. Thank you very much for
getting back to me.
[Edited on 9-1-2018 by JScott] |
Thanks. My intention was to eliminate the need for inert gasses due to the production of CO2. It also reduces the potential for accidents due to the
fact it's not an energetic redox. I hope to try it out myself.
|
|
|
| Pages:
1
..
54
55
56
57
58
..
60 |