| Pages:
1
..
53
54
55
56
57
..
81 |
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Ok thanks PHILOU Zrealone, the Vaseline that i have states no other components except petroleum jelly, it doesn't have a smell or taste so im pretty
confident of its purity, I will try and purchase some proper vacuum grease at some point but for this particular reaction ill try using Vaseline.
I was just wondering if other people used Vaseline for the grignards.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Assured Fish  | Ok thanks PHILOU Zrealone, the Vaseline that i have states no other components except petroleum jelly, it doesn't have a smell or taste so im pretty
confident of its purity, I will try and purchase some proper vacuum grease at some point but for this particular reaction ill try using Vaseline.
I was just wondering if other people used Vaseline for the grignards. |
Then the vaseline is good for the Grignard reaction you intend to do...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
How stable is potassium permanganate / aluminum flash powder (with a touch of fumed silica anticake)?
Need I be concerned about moisture/acid/storage as a finished device?
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
KMNO4 is a quite reactive, powerful oxidizer and there are much safer oxidizers you can use for flash. It likes to react with things (especially hot
fuels) and makes powerful flash even with aluminium powder that won't burn hardly with perchlorate.
It will do if you are very careful with it but there are much safer options.
It has a bit of a bad rap in the flash powder world like acetone peroxide with primaries.
I used to make KMNO4, atomised Al, S flash quite often as a filling for small fire crackers but only a gram at a time, use that and diaper up another
gram.
I never had the finished devices around for longer than a day or two though but they were fine for that.
I wouldn't be exposing it to any moisture though.
If you really must use it be careful of static electricity and friction and make small gram batches that can't self confine and explode.
Be good, otherwise be good at it 
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
I see, thank you, I'll avoid using it then. I was mostly just curious because KMnO4 is so easily available.
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
I'm aware that storing silver fulminate under water is standard practice, but is there any more volatile fluid or solution under which it could be
stored with relative safety and stability? I'm interested in applying a slurry to a surface and having the storage fluid evaporate quickly.
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Are you trying to make a touch explosive of sorts out of it?
I don't think relative safety and stability can be used in the same sentence as silver fulminate. 
It is extremely sensitive and maintains the same sensitivity even if it is wet.
Ammonia will dissolve silver fulminate but when you apply it to a surface, I think it would eventually detonate just from the stress of
crystallization from the solvent evaporating.
I have heard of the weight of crystals on top of crystals causing initiation and add in the fact that bonds forming during crystallization is an
exothermic process. It would be a slower crystallization than the initial precipitation during manufacture which means larger crystals and more
internal stress.
If you did manage to apply it to a surface in dissolved solution, I think it would detonate prematurely.
[Edited on 18-8-2017 by greenlight]
Be good, otherwise be good at it 
|
|
|
physics inclination
Harmless

Posts: 40
Registered: 24-6-2017
Location: the field of physics
Member Is Offline
Mood: thermodynamically stable
|
|
I am NOT planning to try this but...
Since acetylene dissolves in acetone, what would happen if one tried to make TATP from acetylene-enriched acetone? Would the acetylene instantly react
with the hydrogen peroxide? If not, would it get trapped in the precipitate, or bubble out of the acetone as it turns into a solid that can't dissolve
the gas?
|
|
|
physics inclination
Harmless

Posts: 40
Registered: 24-6-2017
Location: the field of physics
Member Is Offline
Mood: thermodynamically stable
|
|
Actually now I have another idea that might not be as dangerous (but still not certain its safe): what if you dissolved nitrocellulose in
acetylene-enriched acetone? Could it make some enhanced-power or higher-detonation-velocity nitrocellulose lacquer? I just remember Darian Berg made a
video about nitrocellulose lacquer that was mixed with Al-KClO3 flash powder and it could detonate, so I wonder if acetylene could do anything
similar, assuming it doesnt bubble out of the acetone when NC is added.
|
|
|
Tin man
Harmless

Posts: 34
Registered: 12-9-2016
Member Is Offline
Mood: Gruntled
|
|
I believe in either case, you would make a flammable liquid.
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
I'm planning on using ETN in order to detonate ANNM (95:5).
Assuming that the main charge (ANNM) weighs about 250g, how much ETN should I use?
What about the blasting cap?
I read something once about ETN that it would react somehow with aluminum and increase its sensitivity in a dangerous level. Would the same reaction
occur with steel?
Regarding the ANNM.
Would a 90:10 mixture make any significant difference (sensitivity, brisance...)?
[Edited on 23-8-2017 by joseph6355]
|
|
|
physics inclination
Harmless

Posts: 40
Registered: 24-6-2017
Location: the field of physics
Member Is Offline
Mood: thermodynamically stable
|
|
So Ive seen lots of good information on metal complex salts that are primary explosives, and I was wondering if there are any similar complex salts
that are more like booster or secondary explosives.
Like, I only need a list of compound names, and I can UTFSE from there 
And sorry to kinda put two questions into one, but this is also related to metal ion complexes: has anyone tried making complex salts with iodate or
bromate as the anion? Both would probably be unstable but I'm just curious.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by physics inclination  | So Ive seen lots of good information on metal complex salts that are primary explosives, and I was wondering if there are any similar complex salts
that are more like booster or secondary explosives.
Like, I only need a list of compound names, and I can UTFSE from there 
And sorry to kinda put two questions into one, but this is also related to metal ion complexes: has anyone tried making complex salts with iodate or
bromate as the anion? Both would probably be unstable but I'm just curious. |
I guess you will find more info than you wish for into the dedicated tread Exotic Primaries - complex salts
and also some into New Energetic Materials - Current Research
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Tin man
Harmless

Posts: 34
Registered: 12-9-2016
Member Is Offline
Mood: Gruntled
|
|
Look into complexes of DMSO and metal nitrates/perchlorates. Fe+3(DMSO)6(NO3-)3 was the only one of the nitrates I could get to precipitate. I did not
try any perchlorates.
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Just a quick question.
I made a small batch of PVA-coated lead azide for primary charge in dets. After I had pressed all I needed I left a small sample out to make sure it
was storage stable. It is now four months later.
The only thing is that whatever part of the pile is in contact with the air becomes darker brown in colour from the original white of the batch and
dearlens more on prolonged storage. I habe noticed this with other batches as well as I only make a small amount at a time.
I have attached a photo below after I transferred it onto a new piece of paper.
Maximum temp it has been exposed to is about 25 C.
Anyone know what causes this or if it affects sensitity or performance?
[Edited on 2-9-2017 by greenlight]

Be good, otherwise be good at it 
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Lead azide left exposed to air and especially to light will get a thin layer of litharge on the surface. Litharge is brown.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
physics inclination
Harmless

Posts: 40
Registered: 24-6-2017
Location: the field of physics
Member Is Offline
Mood: thermodynamically stable
|
|
What is this compound's name, if it has one? I've tried searching under several different names I thought it might be, but couldnt find any instance
of it. Is it known to exist, and if so, is it usefully stable?
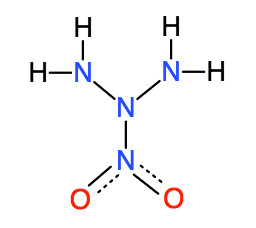
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
@physics inclination
Correct me if I'm wrong, seasoned members, but I believe the name of this compound is 2-nitrotriazane. The compound N3H5 is named triazane
systematically. Assuming the naming is the same as alkanes, the nitro group substitutes the hydrogen on the middle nitrogen and therefore would be
named 2-nitrotriazane.
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by Bert  | Lead azide left exposed to air and especially to light will get a thin layer of litharge on the surface. Litharge is brown.
|
Thanks bert.
Be good, otherwise be good at it 
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by greenlight  | Just a quick question.
I made a small batch of PVA-coated lead azide for primary charge in dets. After I had pressed all I needed I left a small sample out to make sure it
was storage stable. It is now four months later.
The only thing is that whatever part of the pile is in contact with the air becomes darker brown in colour from the original white of the batch and
dearlens more on prolonged storage. I habe noticed this with other batches as well as I only make a small amount at a time.
I have attached a photo below after I transferred it onto a new piece of paper.
Maximum temp it has been exposed to is about 25 C.
Anyone know what causes this or if it affects sensitity or performance?
[Edited on 2-9-2017 by greenlight] |
Pb(N3)2 when left into the air will hydrolyse...via two pathways:
1°) Pb(N3)2 + H2O --> Pb(OH)2 + 2 HN3(g)
2°) Pb(N3)2 + H2O + CO2 --> PbCO3 + 2 HN3(g)
The HN3(g) is toxic/poisonous and volatile just like HCN (cyanhydric acid).
Upon time sensitivity and performance will drop down...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by physics inclination  | What is this compound's name, if it has one? I've tried searching under several different names I thought it might be, but couldnt find any instance
of it. Is it known to exist, and if so, is it usefully stable?
|
As mentionned Ninhydric1... 2-nitrotriazane
or alternatively
==> N,N-diamino-nitramide (nitramide is H2N-NO2)
If it ever exists it will only be as a transient species...even the lower members of the family are not that stable:
-nitro-hydrazine (H2N-NH-NO2) is not stable (adding an extra NH2 will not help at all)
-nitramide (nitro-ammonia H2N-NO2) has limited stability and very fast decompose autocatalytically to H2O, N2 and O2... apparently it is not
stabilised by NH4(+) or N2H5(+) salt formation.
This is to be expected with free nitroazanes...
==> so not "uselfully stable"
Same applies to dinitramide (HN(NO2)2) what display only moderate stability...on its own ...
but here it can be greatly improved by NH3 or N2H4 salt formation...and this comes from the stabilizing effect of the second nitro group...and
resonances structures of the dinitramide anion...
[Edited on 4-9-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
physics inclination
Harmless

Posts: 40
Registered: 24-6-2017
Location: the field of physics
Member Is Offline
Mood: thermodynamically stable
|
|
Quote: Originally posted by PHILOU Zrealone  |
As mentionned Ninhydric1... 2-nitrotriazane
or alternatively
==> N,N-diamino-nitramide (nitramide is H2N-NO2)
If it ever exists it will only be as a transient species...even the lower members of the family are not that stable:
-nitro-hydrazine (H2N-NH-NO2) is not stable (adding an extra NH2 will not help at all)
-nitramide (nitro-ammonia H2N-NO2) has limited stability and very fast decompose autocatalytically to H2O, N2 and O2... apparently it is not
stabilised by NH4(+) or N2H5(+) salt formation.
This is to be expected with free nitroazanes...
==> so not "uselfully stable"
Same applies to dinitramide (HN(NO2)2) what display only moderate stability...on its own ...
but here it can be greatly improved by NH3 or N2H4 salt formation...and this comes from the stabilizing effect of the second nitro group...and
resonances structures of the dinitramide anion...
[Edited on 4-9-2017 by PHILOU Zrealone] |
Thanks for good information, and yea I didnt expect it would be very stable, at least not on its own. But for similar compounds, if anyone's curious,
I do have a few papers saying that some salts and metal complexes of them are somewhat stable:
Attachment: method of synthesizing triazanium perchlorate monohydrate.pdf (256kB)
This file has been downloaded 445 times
Attachment: synthesis characterization and crystal structures of Cu Ag and Pd dinitramide salts.pdf (156kB)
This file has been downloaded 569 times
Attachment: structure and stability of trinitramide.pdf (202kB)
This file has been downloaded 761 times
[edit: added another paper]
[Edited on 9-4-2017 by physics inclination]
Attachment: synthesis and modes of coordination of energetic nitramine ligands in copper 2 nickel 2 and palladium 2 complexes.pdf (1.7MB)
This file has been downloaded 466 times
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by physics inclination  | Quote: Originally posted by PHILOU Zrealone  |
As mentionned Ninhydric1... 2-nitrotriazane
or alternatively
==> N,N-diamino-nitramide (nitramide is H2N-NO2)
If it ever exists it will only be as a transient species...even the lower members of the family are not that stable:
-nitro-hydrazine (H2N-NH-NO2) is not stable (adding an extra NH2 will not help at all)
-nitramide (nitro-ammonia H2N-NO2) has limited stability and very fast decompose autocatalytically to H2O, N2 and O2... apparently it is not
stabilised by NH4(+) or N2H5(+) salt formation.
This is to be expected with free nitroazanes...
==> so not "uselfully stable"
Same applies to dinitramide (HN(NO2)2) what display only moderate stability...on its own ...
but here it can be greatly improved by NH3 or N2H4 salt formation...and this comes from the stabilizing effect of the second nitro group...and
resonances structures of the dinitramide anion...
|
Thanks for good information, and yea I didnt expect it would be very stable, at least not on its own. But for similar compounds, if anyone's curious,
I do have a few papers saying that some salts and metal complexes of them are somewhat stable:
|
Nice articles, thanks.
I really like the triazane perchlorate one...
Too bad into most characterization they don't give interesting datas for detonics properties... except density... 
==> Heat of formation, Enthalpy of decomposition, Calculated VOD, Temperature of decomposition, Impact sensitivity, ...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by greenlight  | Just a quick question.
I made a small batch of PVA-coated lead azide for primary charge in dets. After I had pressed all I needed I left a small sample out to make sure it
was storage stable. It is now four months later.
The only thing is that whatever part of the pile is in contact with the air becomes darker brown in colour from the original white of the batch and
dearlens more on prolonged storage. I habe noticed this with other batches as well as I only make a small amount at a time.
I have attached a photo below after I transferred it onto a new piece of paper.
Maximum temp it has been exposed to is about 25 C.
Anyone know what causes this or if it affects sensitity or performance?
[Edited on 2-9-2017 by greenlight] |
As others stated it is decomposing to lead oxide
For long term storage stable caps your components must be well dried and the cap hermetically sealed, as must your primaries!
A well dried cap is a very long storage stable cap!
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
There is considerable information available for high explosives based on mixtures of Potassium chlorate and nitrobenzene or of ammonium nitrate and
nitromethane.
Do any know of data on mixtures of Potassium chlorate and nitromethane? VOD, impact and friction sensitivity? (high, one assumes...) STABILITY? Was
there ever any industrial use made of this mixture?
A quick Google search shows some amateur experimental uses, and at least one video.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
| Pages:
1
..
53
54
55
56
57
..
81 |