| Pages:
1
..
53
54
55
56
57
..
77 |
JnPS
Hazard to Self
 
Posts: 90
Registered: 29-7-2016
Location: PA, USA
Member Is Offline
Mood: Umpolung
|
|
I loved the green color of chlorophyll dissolved in ether, also this is a picture of the biggest copper sulfate crystal i have grown to date, the
picture's a bit bad but I have a video showing off the crystal on twitter, and the last photo is my recrystallized paracetamol crystals ^.^
  
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
That is some clean-looking paracetamol!
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|

Accidental 'hot ice'! Cool looking crystals after boiling down sodium acetate solution. Brown/yellow(ish) color is from organic impurities, thanks to
the vinegar I used making it.

Woah, the top turned white while I was writing this.

And after breaking up
[Edited on 2/6/2017 by Geocachmaster]
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
That white crystals on second pic may be leftover sodium carbonate if stoachiometric amounts weren't used.
The same thing sa as interfering my first try on hot ice as it wouldn't let the solution to supersaturate.
Nice pics by the way  . .
[Edited on 6-2-2017 by crystal grower]
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
I don't think it was sodium carbonate because I used an excess of vinegar; I'd rather suffer through boiling acetic acid into the room than have
contamination. I'll check later for any carbonate contamination though.
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Quote: Originally posted by Geocachmaster  | | I don't think it was sodium carbonate because I used an excess of vinegar; I'd rather suffer through boiling acetic acid into the room than have
contamination. I'll check later for any carbonate contamination though. |
I may be wrong, it just resemble me my experiment.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by Amos  | Copper(II) formate crystals. Their supernatant is the deepest of royal blues and yet the crystals are an icy-looking aqua color.
 |
Hi Amos, how did you prepare it? Just dissolved (basic) copper carbonate in formic acid?
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by Bezaleel  | Quote: Originally posted by Amos  | Copper(II) formate crystals. Their supernatant is the deepest of royal blues and yet the crystals are an icy-looking aqua color.
|
Hi Amos, how did you prepare it? Just dissolved (basic) copper carbonate in formic acid?
|
Yep, simple as that. The crystals form lightning fast if you leave a saturated solution of it alone, very easy.
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Reduction of KMnO4
There is nothing more useless than doing well that which need not be done at all.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Dwarvensilver, looks like you reduced it all the way down to nothing!
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Yep, massive reduction  . .
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
1 L Flask with lots of prussian blue on the bottom.

95% of the stuff I do is based on transition metals and I'd say 40-50% is cyanide based. So automatically blue Cyano-Iron phases like prussian blue
should form when the wastes are combined.
But there is another reason why this is so much. I use this flask for the wastes I produce in the lab. It is in an old aquarium in case it leaks or
breaks and contains a month's or two months' amount of waste to settle. Added is always some Ferri(o)cyanide, DMG, Hydroxides etc to bind most of the
wastes. It settles down and only the clear solution is decanted off into secondary containers. So in case one of the waste bottles breaks there isn't
much heavy metal in there. So jus working with Iron salts will produce a lot of prussian blue in the end but it looks quite nice. Used to be a blue
solution and now after a few months it is all settled.
|
|
|
anewsoul
Harmless

Posts: 32
Registered: 26-1-2017
Member Is Offline
Mood: No Mood
|
|
Here's some nice crystals of phthalic anhydride I got from a recrystallization.

|
|
|
Supersonic
Harmless

Posts: 3
Registered: 2-3-2017
Member Is Offline
Mood: God
|
|
Quote: Originally posted by Amos  | Copper(II) formate crystals. Their supernatant is the deepest of royal blues and yet the crystals are an icy-looking aqua color.
|
Large crystals have a nice form and beautiful color.
It`s a pity that they erodes even in warm air.
There crystals were synthesed from copper(II) hydroxide and formic acid.
   
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Another try to film the mysterious blue-green gas in potassium reactions:
http://imgur.com/zCVaKoA
For a while now I filmed dozens of reactions involving potassium in other solvents besides water to slow down the reaction it usually shows.
Originally Thunderf00t pointed out that in the reaction with water under inert gas atmosphere a blue gas forms.
I looked at so many of these reactions now and quite often this blue-green gas really is involed in the reaction. It leads me to believe that maybe
potassium reacts or more burns from a gas phase and not as an element. I've seen for example quite often two differently colored flames in narrow and
high flasks one that burns longer way above the metal which should be hydrogen and then a pale flame with the color of potassium which burns or more
glows around the metal as it becomes smaller and smaller in water.
Funnily whenever I add an oxidized sample the water or acid would first react with the upper layer making it a shiny oxide-free ball. But always
shortly before and after it either combusts or starts to melt, glow, burn etc. I've noticed the metal becoming either dark on its surface or form a
blue-green gas around the metal itself which then burns instead of the metal. And I've checked this in literature as well while a K2 is
very unlikely at these temperatures small nano-potassium particles are blue, too. So maybe the potassium reacts by forming fine nano particles which
then react to fast that it appears like a burning cloud around it.
In the picture you can see a small lump of potassium which suddenly (1/30th of a second) turns all black then reacts (note that the gas burns above
the metal) and afterwards shows a green-blue gas (some frames later this gas catches fire and the whole thing starts to glow).
I've had better ones before maybe I'll make a small collection of all these gases and upload it here.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Hopefully some RuO4 (video will come soon on YT).
Distilled some RuCl3 with H2SO4 and KMnO4.

|
|
|
A Halogenated Substance
Hazard to Self
 
Posts: 68
Registered: 7-2-2017
Location: United States
Member Is Offline
Mood: Oxidizing due to extended exposure to oxygen
|
|
I got a spectrometer and decided to compare the emission spectrum of mercury with that of a fluorescent lightbulb. Looked like a pretty good match to
me!
 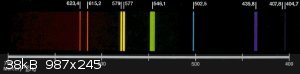
Current Active works in Progress:
-Convert i-propanol to i-chloropropane using HCl and ZnCl2
-Determine properties and solubility of a glycerol and citric acid polymer
-Attempt a conversion of chloroform to dichloromethane
-Attempt a conversion of acetic acid to acetamide
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Guess how i made this RB flask scrap?

[Edited on 18-3-2017 by Waffles SS]
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Not fully reacted yet but a mixture of
[RuVIO2Cl4]2- and [RuIVOCl10]4-

|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Yet uncharacterized Dy complex salt at 10x magnification.
<img src="http://chem.pieceofscience.com/wp-content/uploads/2017/03/Image_5924.png" height="400" width="600">
Microcrystals of probably new copper benzoate complex.
<img src="http://chem.pieceofscience.com/wp-content/uploads/2017/03/Cu-benz3.png" height="400" width="600">
Laue diffraction pattern of ErFeO3 monocrystal oriented along the c-axis.
<img src="http://chem.pieceofscience.com/wp-content/uploads/2017/03/VI000034.jpg" height="500" width="600">
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Diborane 
  
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Recrystallized ammonium chloride:

The hair-like towers continued to grow with time, looking a lot like sodium silicate crystal gardens:

Ice crystals in the parking lot after winter storm Helena:

al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Reaction of what I believe is KSeCN (not sure so something between K2Se, KHSe and KSeCN reacting with a drop of conc. HCl. You can see how
it takes around 0.1 seconds for the redox reaction to happen, quite interesting how slow this is. The bottom pictures were how it looked after a few
additional drops.

|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Selfmade OsO4 solution (left), took me a while to find a chemical that reacts with solid Os metal and then reduced Potassium Osmate(VI) in
Ethanol (right) 

|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Some Cu(bpy)2(Cl)26.8H2O recrystallised from acetone and water

[Edited on 19-4-2017 by HeYBrO]
|
|
|
| Pages:
1
..
53
54
55
56
57
..
77 |