| Pages:
1
..
52
53
54
55
56
..
77 |
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
have you looked carefully at the crystals ?
I recently re-crystalised salicylic acid from water just to see the hollow square crystals.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
Salicylic crystals are hollow?! That's weird. Upon closer examination they are square, but I can't tell if they're hollow. Perhaps I'll have to take a
look under the microscope.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I could see the 'hollowness' using a magnifying glass but I could not get a decent photo' with my 'phone, hence not shown before.
this is the best photo' that I got ... growing in a beaker

CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Mabus
Wiki Master
  
Posts: 238
Registered: 3-11-2013
Member Is Offline
Mood: Energetic
|
|
Some NaCl crystals I grew last year. I placed 2 L of supersaturated NaCl solution in a wide plastic tray and left it to evaporate over the summer.
When the volume decreased to half, I noticed the crystals grew much slower, so I decided it wasn't worth the effort and simply took them out of the
solution. The next batch gave smaller crystals, I guess the bigger the solution volume, the bigger the crystals. Oh and the NaCl I used was from water
softening tablets.

|
|
|
chironex
Harmless

Posts: 40
Registered: 17-10-2016
Member Is Offline
Mood: No Mood
|
|
Some of my favorite pictures. I know they're not all strictly chemistry, but I thought people here could still appreciate them:
hydrothermally grown carbon nanofoam SEM:

DC magnetron I built:

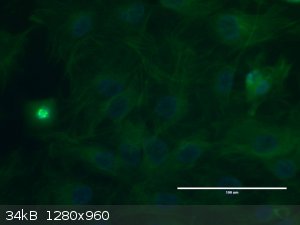
Immunoflourescently labeled ad DAPi stained mouse 3t3 cells, 1 undergoing mitosis:
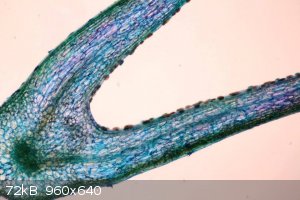
Venus fly trap, trap, stained with tuluidine blue. Digestive enzyme excretion glands can be clearly seen:
Decellularized pig heart and decellularized strawberry:
 
And finally, hydrothermally grown copper crystals:
 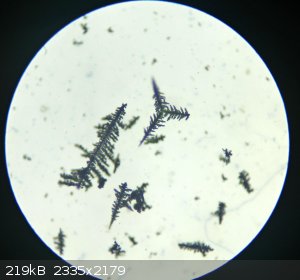
|
|
|
Texium
|
Thread Split
5-1-2017 at 10:39 |
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|

Iodine crystals. And a bit of tiny ember glass shards.
[Edited on 6-1-2017 by TheMrbunGee]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Wow, MrbunGee, those iodine crystals are really neat! I've never seen any that look quite like that. How did you go about making them?
Also, I have my own pretty crystal picture to share now. I found these in the container of waste acetone that I used to rinse off my glassware after
making Martius yellow (2,4-dinitronaphthol). I wouldn't have expected Martius yellow to grow such nice crystals, but it did!

I'd like to try growing a larger one now that I know it works.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by zts16  | | Wow, MrbunGee, those iodine crystals are really neat! I've never seen any that look quite like that. How did you go about making them?
|
It came from 40 year old hydrogen iodide bottle, the HI had oxidized and most of the iodine crystalized out very slowly!
Here is the video of getting it out!
https://www.youtube.com/watch?v=4LMd8xiq-xY
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Definitely to nice to brake up that iodine!
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
A so called Metalcarbonylhydride or Hieber-Base, in this case K[HCr(CO)5]. Had to prepare this at the university this week. It's quite
unstable but has a lovely color.

|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Lovely video. I am going to have to watch more of those.
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Cemented copper from e-waste sol, onto a large square nail (~12" long). It nearly ate through at the liquid/atmosphere interface.
  
Manganese sulfate from battery crud. The reddish was from the first pull, second go was much lighter colored. What a mess to play with. Brought
back memories of much earlier experiments with copper.
 
Alum from a heat-sink/H2SO4. I had none laying around, but did have the makings for it. The stuff was heated untill a light
crust formed, then cooled. It had a really cool gell texture with an opalescent marbled look. Neat

Zinc sulfate crystal in petri dish.
[Edited on 14-1-2017 by violet sin]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
A crystal of sucrose, a crystal of nickel-doped ammonium magnesium sulphate dodecahydrate, and crystals of bis(ethylenediamine)copper(II) nitrate.
  
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
How does one get so clear transparent crystal? Mine are always dim.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The crystal of sucrose, sadly, was not intentionally grown, but was found in a very old bottle of children's cough syrup.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
CaCl2
Harmless

Posts: 39
Registered: 14-1-2017
Member Is Offline
Mood: No Mood
|
|
Some copper compounds.
https://imgur.com/a/nPxHC
Details here: https://www.sciencemadness.org/whisper/viewthread.php?tid=71...
[Edited on 14-1-2017 by CaCl2]
[Edited on 14-1-2017 by CaCl2]

|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Love the rainbow display, cool stuff ^^^
1) Flea market score. 1$/ea
2) View of a family members front deck 
 
They were doing cedar essence oil from chips and needles.
[Edited on 15-1-2017 by violet sin]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
On the left, Martius yellow as the ammonium salt in aqueous solution, and on the right as the free acid upon addition of a few drops of conc. HCl.
 
Not sure why they're showing up sideways.
Oh yeah, and aga, I realized that if I set my phone camera to "square," the pictures are just small enough to post here, so I will likely double post
more of my pictures between my blog and here.
https://texium.wordpress.com/2017/01/17/update-on-martius-ye...
|
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
Tin(ii) oxalate that I just made.
I find the needle-like crystal structure quite beautiful.

reality is an illusion 
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Looks like snow  or coconut dust or coconut dust 
What intend you to do with that SnC2O4?
What will it be used for?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
gluon47
Hazard to Self
 
Posts: 81
Registered: 20-9-2015
Location: oceania
Member Is Offline
Mood: fluorinated and dying
|
|
Yeah, It really does look like coconut dust now you say it , not so much in real
life though. , not so much in real
life though.
I plan to convert it to tin(ii) oxide by heating with ammonia solution, then try isolating elemental tin via thermite . .
[Edited on 24-1-2017 by gluon47]
reality is an illusion 
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Efflorescence of magnesium sulfate
A c150 mm tall x 50mm dia porous pot that was in a saturated epsom salt solution, drained, and left for over a year in a plastic tub.

CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Dwarvensilver
Hazard to Self
 
Posts: 52
Registered: 8-6-2016
Member Is Offline
Mood: Constantly Chemically Amazed
|
|
Wow Sulalman, that is awesome! Beautiful and something you don't see every day for sure.
There is nothing more useless than doing well that which need not be done at all.
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Sulaiman, nice pic. I find some pretty cool things happen when stuff is left alone for a long time. Too bad it is usually hard to photograph
withough wrecking it. In the past I have had real neat crystals form in old solution, only to kick crud all over them when moving the beaker for a
pic.
Glad you were able to share with out messing it up
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Copper(II) formate crystals. Their supernatant is the deepest of royal blues and yet the crystals are an icy-looking aqua color.

|
|
|
| Pages:
1
..
52
53
54
55
56
..
77 |