| Pages:
1
..
3
4
5
6
7
..
25 |
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
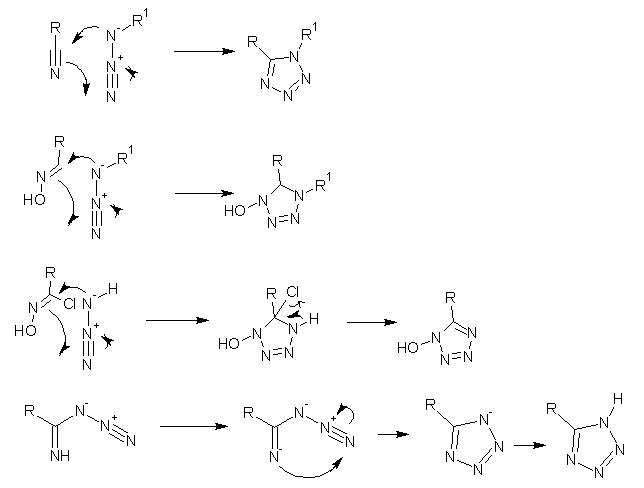
I do not know how much you have looked at the synthesis of furazans(https://sciencemadness.org/talk/viewthread.php?tid=5813), but the intermediate for the synthesis of diaminofurazan is diaminoglyoxime.
Diaminoglyoxime is synthesized from glyoxime and hydroxylamine HCl. I believe the OH of hydroxylamine is necessary in the mechanism of hydroxylamine
reacting with glyoxime. Without it there is no group to leave, and the mechanism will not work. if a HON3 compound existed, it would be a good one
to try in this reaction.
If I am wrong here, and the azide did add somehow to the glyoxime, I cannot see it easily cyclizing, unlike the reaction of a nitrile with an azide,
the tetrazole ring is not formed immediatly, a nonaromatic system is formed, and an OH is left on the nitrogen, and there are no simple methods for
elimination forming the ring. See the attachment for what I mean.
However if R1 on the azide is a hydrogen or just a negative charge, this could be used to make the tetrazole ring, but only if dichloroglyoxime was
used instead of glyoxime, as it would provide a nice leaving group for elimination making the tetrazole ring. But the OH group may be left intact....
In the most recent scheme you posted, I think it could work, or at least I could draw a mechanism by which it could easily work in the presence of a
strong base.
[Edited on 28-3-2008 by The_Davster]
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by The_Davster
dichloroglyoxime was used instead of glyoxime, as it would provide a nice leaving group for elimination making the tetrazole ring. But the OH group
may be left intact....
|
Arr confirmed, I posted it in the "Glyoxime, Diaminofurazan and Some Energetic Derivatives" before realising the discussion here. http://www.sciencemadness.org/talk/viewthread.php?tid=5813
I'll attach a previous article regarding the diazidoglyoxime intermediate here, although these authors failed to cyclise the azide to tetrazole. See
the link above for the cyclisation via HCl in ether.
[Edited on 24-5-2008 by Axt]
Attachment: Evidence on the Existance of Azidoximes - Journal of Organic Chemistry (1961), 26 952-4.pdf (348kB)
This file has been downloaded 1791 times
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I like!
I wonder if 5-ATZ would react with dichloro glyoxime, which could then be formed into a tetrazole substituted furazan?
I think the tetrazole ring would be stable under the ring closing conditions required for making a furazan.
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Finaly i got all chemicals i need for synth of DAT (1,5-diaminotetrazole), i found reference on path from thiosemicarbazide via desulfurization and
reaction of intermediate with ammonium azide in dimethylformamide solution:

Synth from reference, attached to this message (translated from russian): 18g (0.2 mol) thiosemicarbazide, 16.3g (0.25 mol) sodium azide, 13.4g (0.25
mol) NH4Cl and 89.2g (0.4) PbO are mixed in 350 ml of dimethylformamide on boiling water bath for 6 hours. Mixture is filtered hot, and filtrate is
evaporated to dryness in vacuum. Residue is dissolved in 50 ml of hot water, filtered hot and slowly cooled. Precipitate is filtered, washed with cold
water and dried. Yield is 11.8g (59%), white crystals, melting point 186-187C with decomposition (from water). Good soluble in hot water, and
water-ethanol mixtures, acids, dimethylformamide, moderately soluble in cold water, ethanol, insoluble in tetrahydrofuran, ethylacetate, methylene
chloride, either.
However, side reaction of HN3 and PbO surely exists and some lead azide will also form.
Question is how safe is to boil this miture on water bath? Do anyone have references on energetic materials whitch can be made from DAT? (I heard that
some complexes such as with Fe(ClO4)2 are proposed as lead azide replacement primaries). Any known properties of DAT salts with strong acids?
[Edited on 12-6-2008 by Engager]
Attachment: diaminotetrazole.djvu (61kB)
This file has been downloaded 1572 times
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Nitrosation of diaminotetrazole. There was only five (prepared and isolated) structures in the scifinder database that had a string of 7 nitrogens, at
least that I could see. Of which only two looked notably energetic, this being one and <a
href="http://www.sciencemadness.org/talk/viewthread.php?tid=10555">this</a> the other.
Personally I wouldn't have a problem heating a small amout of Pb azide dispersed in a solvent to 100°C, unless it wanted to bump and splutter but
that shouldn't be a problem with DMF. Patent SE154979 precipitates Pb(N3)2 from Pb(NO3)2 + NaN3 at 90°C, a more extreme example is US3345130 which
precipitates it from molten KNO3/NaNO3 at 225°C!! (Pb(N3)2's decomposition temp is >300°C).
[Edited on 13-6-2008 by Axt]

|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
And an article on diaminotetrazole perchlorate.
Attachment: Experimental and Theoretical Study of 1.5-Diamino-4-H-Tetrazolium Perchlorate.pdf (1.2MB)
This file has been downloaded 1662 times
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I imagine it would have a plethora of interesting condensation products with formaldehyde or glyoxal. Unfortunatly I do not know what work has been
done on this, I will research it at work.
As for the lead azide, I have heard (High Energy Density Materials, Klapoetke) that this synthesis will produce "large ammounts" of lead azide, and
that is why the method from diaminoguanidine is prefered. But this may only be important on industrial scale.
DMF boils at a higher temperature than a boiling water bath, and should be safe from thermal issues. I would advise swirling to mix the reaction as
opposed to magnetic or hand stirring to keep friction to a minimum.
Also, the Indian paper Axt posted...an isomer of azidotetrazole/tetrazyl azide! I could have sworn I have seen a paper on interconverting the two, or
was it a theoretical discussion paper?
Anyway, in terms of derivitaves, the nitrate and perchlorate are in the attached paper, as are the methylation products with azide, nitrate, and
dinitramide counterions
Attachment: DAT derivs.pdf (493kB)
This file has been downloaded 2617 times
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
A paper on DAT condensation with glyoxal... reactions with formaldehyde appear nonexistent.
Attachment: DAT condensations.pdf (572kB)
This file has been downloaded 1840 times
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Reciently i made some biguanide sulphate, and got an interesting idea, about how another interesting tetrazole can be made. How does it look? Someone
have synth procedure for biguanidine or it's salts (first made by Thiele in last century)?

|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I can find no problems with the synth, and I REALLY like the idea.
The molecule has to be some sort of a record...8 nitrogens connected in a row .
I think the diamino version may be explosive by itself, let along the dinitro one .
I think the diamino version may be explosive by itself, let along the dinitro one 
|
|
|
Sobrero
Harmless

Posts: 21
Registered: 20-5-2006
Location: Belgium
Member Is Offline
Mood: Lifting my skinny fists like antennas to heaven
|
|
Perhaps dinitrobiguanidine can be made by reaction of fuming nitric acid (or mixed acids) with biguanidine sulphate, just like nitroguanidine can be
made from guanidine sulphate + HNO<sub>3</sub>. This would shorten the synthesis somewhat  . .
"There exists a world. In terms of probability, this borders on the impossible." (Jostein Gaarder)
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
God that is a lot of nitrogens. I like it. Any practical information on the synth or explosive properties?
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
Even looking at it scares me a little bit, but yeah, I like it  . .
The only potential problem that I can see could be acid hydrolysis of some of the intermediates, eg dinitrobiguanidine into N-amino-N'-nitroguanidine
and nitrourea.
It's definitely worth trying though!
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
A couple of thoughts on first look...
will have to dig through some references later....
There may be a possible shortcut from the biguanidine
sulfate to the diaminobiguanidine via reaction with hydrazine, ammonia being byproduct.
Possibly a variation on the reaction for producing guanidine salts via calcium cyanamide and ammonium nitrate might preferentially produce the
biguanidine salt.
Polymerization to undesired materials could be a real complication, too. Beware the red goo
[Edited on 16-7-2008 by Rosco Bodine]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Some references on biguanidine:
1. Justus Liebig's Annalen der Chemie, Volume 273, Issue 2-3 (p 133-144). J.Thiele "Uber Nitrosoguanidin" ; http://www.sciencemadness.org/talk/viewthread.php?action=att...
2. Justus Liebig's Annalen der Chemie, Volume 270, Issue 1-2 (p 1-63) J.Thiele "Uber Nitro And Amidoguanidine" ; http://www.sciencemadness.org/talk/viewthread.php?action=att...
Corresponding English translations:
3. http://www.rsc.org/delivery/_ArticleLinking/DisplayArticleFo...
page 389 J. Thiele Nitrosoguanidine
4. http://www.rsc.org/delivery/_ArticleLinking/DisplayArticleFo...
page 1295 J.Thiele Nitro and Amidiguanidin
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
guanyl azide (diazoguanidine) revisited
In the second article above is mentioned again a material of interest, diazoguanidine, as the nitrate m.p. 129C which was also identified near the end
of page 2 as the picrate in the early part of this thread. A parallel British patent GB470418 by the same inventor gives a little more detail about
the preparation of the picrate. Analogously, by using half the molar proportion of magnesium styphnate, a guanylazide styphnate would be expected.
And likewise using the sodium or ammonium salt of some of the acidic tetrazoles, the guanyl azide salt of those would be expected. Some of these may
form mixed metal salts of possible interest also.
And there may be a shortcut to the aminoguanidine precursor from reaction of the ordinary guanidine nitrate salt with hydrazine. And likewise the di
and triaminoguanidine bases via this and other routes should be kept in mind as possibly useful in forming salts with the acidic tetrazoles.
Anyway, aside from the new compounds proposed by Engager, there is a reminder there in the readings which
also points back to the beginning of the thread and these
other possibilities. It seems like deja vu all over again
@Engager , in that next to the last reaction above, instead of
Na2CO3 for the base, you should use NH4OH. Right?
[Edited on 17-7-2008 by Rosco Bodine]
Attachment: GB470418 Guanyl Azide Picrate base charge.pdf (265kB)
This file has been downloaded 1949 times
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Yesterday i decided to try another alternative route to bis-tetrazole. It is well known that it simply forms on action of nitrous acid on oxalic bis
amidrazone (see attached patent for example). Main problem with less complicated synths is that they require to work with highly toxic dangerous gas
cyanogen. As i wrote in posts before, attempts to react glyoxime with hydrogen azide, and additon of HNO2 to mix of glyoxime and hydrazine vere
unsuccessful, however i decided to try another route:

First i made ammonium oxalate from ammonia and oxalic acid:
39g of oxalic acid dihydrate was dissolved in 60 ml of water at 60C, solution is quickly filtered to the flask containing 54 ml of 25% ammonia
solution. White precipitate forms imidately, solution is cooled to 25C to further reduce product solubility, and then filtered. Resulting white
glistering crystalls of pure ammonium oxalate monohydrate were filtered and air dried. Yield is 34.1g (77.5%).
Ammonium oxalate was converted to oxamide by following method:
28.4g of ammonium oxalate monohydrate is ground to fine state and mixed with 0.66 of P2O5 (catalyst) and wetted with 20 ml water. Mixture is placed to
round bottom flask with attached air condenser, joined with distillate flask (to keep condensed water escaping from reaction mixture) and gas outlet
(to vent toxic side products such as CO or (CN)2 outside of building). All connections must be tight to ensure no toxic reaction gasses can enter room
atmoshpere. Flask with reaction mixture is heated on wax bath to 200C for 2 hours. Solid residue was washed with 2 portions 250 ml of hot water to
remove soluble impurities and then filtered to obtain insoluble product - oxamide with 63% yield.
Next i tried to condense resulted oxaimide with hydrazine salts in water and alcohol. Because oxalic bis amidrazone is unstable at temps more then 50C
heating can not be applied to help this reaction attempts. Hydrazine salt was used because according to information from the books amidrazones are
stable in oxidic conditions and unstable in alkaline, however no reaction accured at all - oxamide does not dissolve. Then other metod is tried, some
N2H5Cl is dissolved in ethanol and freebased by sodium hydroxide. Oxamide was fast dissolved and solution was filled with white flakes of product,
whitch is insoluble in alcohol, probably oxalic bis amidrazone =)
[Edited on 18-7-2008 by Engager]
Attachment: EP1136476B1.pdf (207kB)
This file has been downloaded 1479 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
You might try Copper Acetate in mild acetic acid at pH 4 and add Sodium Nitrite for the diazotization of the oxalic bis-amidrazone (oxamide
dihydrazone) and simultaneous conversion to the energetic copper salt of the BHT as the end product precipitate.
Also the use of Copper Nitrate in dilute HNO3 with NaNO2
would probably work parallel to the prior art example 1,
analogous to the silver salt. Other metal salts should form similarly, shortening the synthesis if the metal salt is what is wished to be the end
product.
You might want to have a robot do the work while
you remain safely behind a barricade
Attached is the US7122676 filing for the same patent which is easier to navigate.
[Edited on 19-7-2008 by Rosco Bodine]
Attachment: US7122676 preparation of 5,5′-bi-1H-tetrazole.pdf (115kB)
This file has been downloaded 1764 times
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
"1 H-Tetrazolo[1,5-d]tetrazole: A solution of sodium nitrite (0.01 mol) in water (5 ml) was added dropwise to a stirred solution of compound
diaminotetrazole (0.01 mol) in conc. HCl (5 ml). The reaction mixture was kept at 0°C for 30 min and the precipitated solid was filtered, washed with
water, dried and crystallized from ethanol. Yield 0.6 g (55%), m.p. 210°C." J. Ind. Chem. Soc. 82, 172-174, (2005) [attached].
Attachment: Use of 1,5-diaminotetrazole in the synthesis of some fused heterocyclic compounds.pdf (263kB)
This file has been downloaded 2237 times
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
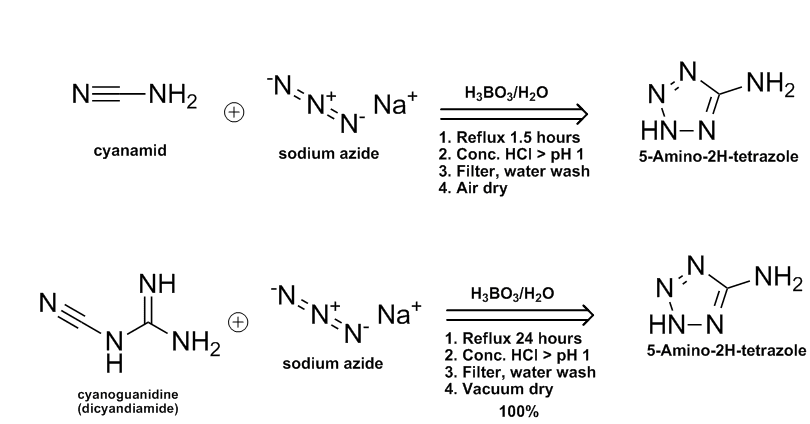
5-Aminotetrazole on eBay: item 300165803620. $70.50 for 500 gm of 97.0% min. material.
Ref US 5451682 http://tinyurl.com/6hoacl & ChemDraw abstract of 2 azide routes to 5-AT from either cyanamid or dicyandiamide.
[Edited on 30-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Synth of DAT has been attempted using procedure i've mentioned somethere above, however some serious difficulties has been encountered. Insoluble
product after boiling tends to be rock hard, and if it contains significant ammount of lead azide this could result in hazard situation then attempted
to separate this black sludge from glass vessel. Liquid was decanted and insoluble black residue on walls of flask was successfully destroyed without
problems by long standing with weak nitric acid. Particles of PbS are very small and it is very hard to filter them completely, to get transparet
solution whitch has dark orange color.
Another more serious problem has arisen on evaporation of DMF. At boiling water bath in vacuum made by water-stream vacuum pump, DMF is not destilled
with any visible speed. Removal of DMF at ordinary pressure is imposible in this case because DMF boiling point is to close to DAT decomposition
temperature. It is quite interesting to know that is b.p of DMF in vacuum about ~50 mm. Hg. How to solve solvent removal problem?
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
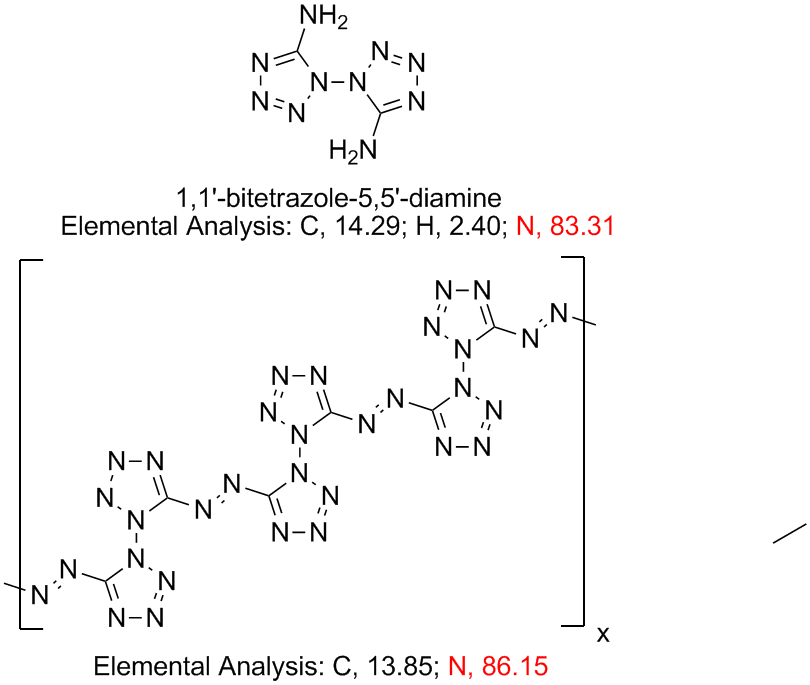
| Quote: | Originally posted by Engager
Reciently i made some biguanide sulphate, and got an interesting idea, about how another interesting tetrazole can be made. How does it look?
 |
I'm curious to find out if this diamine can be oxidized to create multiple intermolecular azo linkages, basically polymerizing it into a polymer that
would be anout 86% nitrogen.
The synthesis of 1,1'-dinitro-3,3'-azo-1,2,4-triazole uses alkaline permanganate to oxidize the amino function to the azo group.
It would be poly(5,5'-azo-1,1'-bitetrazole) or thereabouts. Are high nitrogen polymers of this type known or even possible?
[Edited on 30-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Strange effects observed during attemp to saparate DAT from reaction mixture. First of all i tried to precipitate DAT with tetrahydrofuran (dat is
insoluble or weakly soluble in it), this resulted in no precipitate; same thing happens then ethylacetate is added.
Next thing that was tried is benzene, if it is added in large excess to DMF in reaction mixture (3-4 times vol) mixture orange color deminishes and
some creme colored product is precipitated, it is glueish like and can not float free in solution. This product is isolated with filtration (product
№1), when filtrate is allowed to stand for some time formation of strange brown oily layer is observed, most of liquid is decanted and oily
layer is collected by means of separating funnel (product №2). Then brown oil with strange angry smell is dilluted with water (1:1 vol) large
quantity of white product is separated momentaly and color discharges to very slightly yellowish transparent, product is separated by filtering
(product №3).
Some observations on products: product №1 is good soluble in hot water, however small quantity of insoluble materal remained. Then a drop of
this solution is added to drop of CuSO4 solution, this results in imidate formation of brown precipitate. It is most likely that №1 product is
traces of unreacted material - inorganic salts, whitch are weakly soluble in benzene, may be containing some azide. Product №2 is very strange,
benzene and DMF are miscible with each other in all possible volume rates, so formation of this layer remains mysterious. This brown oil is heavier
then mixture of benzene with DMF so it flows on the bottom.
My first idea about that layer was that it is layer with tetrazylazide whitch can form as byproduct in synth of DAT as mentioned in
(5-Azido-1H-tetrazole - Improved Synthesis, Crystal Structure and Sensitivity Data article)
http://www.sciencemadness.org/talk/viewthread.php?action=att...
Synthesis of tetrazylazide mentiones about it's extraction from some brown oily layer, as described in this reference:
http://www.sciencemadness.org/talk/viewthread.php?action=att...
But i have to revise it because 5-azidotetrazole and it's alkali metall salts are perfectly soluble in water, so this product is likely to be DAT
because it is weakly soluble in cold water. However i'm not completely sure in it.
Any ideas what all this products could be? How to explain this observations? May be someone know qualitive tests for DAT?
I've attched some pictures of this mysterious brown oil, and precipitate it forms on dilution by water:
 
[Edited on 31-7-2008 by Engager]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Maybe filter and dry the precipitate gotten from dilution with water.
Redissolve in alcohol and precipitate with methylene chloride to see if crystallization occurs.
I think you probably have DAT there as the white
precipitate from dilution with water, pure enough for use
as is, for further reactions like nitrosation as Axt described, or for permanganate oxidation
and possible polymer formation as Ritter speculated above.
@Ritter
Please edit your attached images width to ~600 pixels so the images don't foul up the text formatting
on the page. The width will be the first pixel dimension stated in the image properties, to resolve
any confusion about which number there specifies the width.
[Edited on 30-7-2008 by Rosco Bodine]
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Rosco Bodine
@Please edit your attached images width to ~600 pixels so
the images don't foul up the text formatting on the page.
[Edited on 30-7-2008 by Rosco Bodine] |
??? 810x685 pixels for that image. Looks fine to me. Do you prefer 600x600 or ?x??
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
| Pages:
1
..
3
4
5
6
7
..
25 |