| Pages:
1
..
3
4
5
6
7
..
22 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK.
Thanks for the clarification.
It'll stay here then - hopefully with at least 1 alternate route to HNO3 !
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I am curious if anyone has had any thoughts about converting ammonia to nitrite by nitrosomonas, and then nitrite to nitrate using nitrobacters?
I think this can actually be done using pool/water filters. It would be a very slow process, and would likely have to be done on a large scale, but
nitrates are eventually produced.
http://en.wikipedia.org/wiki/Nitrification#Chemistry
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Quote: Originally posted by Loptr  | | I am curious if anyone has had any thoughts about converting ammonia to nitrite by nitrosomonas, and then nitrite to nitrate using nitrobacters?
|
It crossed my mind. Technically I have aquariums converting it right now. It would be a pain to get significant quantities, though.
[Edited on 1-24-2015 by Etaoin Shrdlu]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Ok, here is another idea.
What about oxidation of dichloramine (NHCl2)? Chloramines are typically something you want to stay away from, not to mention possible formation of
NCl3. Chloramines can be formed from mixing hypochlorite bleach and ammonia, so that makes dichloramine almost within reach and off-the-shelf?
https://books.google.com/books?id=8cv36t8mOEwC&pg=PA201&...
[Edited on 24-1-2015 by Loptr]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Well, today is the day -- if my memory serves me correctly.
I am genuinely surprised to find that the submissions are so... um... nonexistant. There have been a large number of good ideas floated. None
without certain difficulties, but that is the nature of the competition.
I doubt I was ever in the running myself. I just don't have the distillation rig needed to attain the required volume and concentration -- at east
not without a larger expense of time than I can afford. I was hoping to do a proof of concept though -- or disproof of concept as the case may be.
As it turns out, I achieved neither. I am not in a position to write off my idea as totally worthless but neither did I achieve any positive results.
For the sake of posterity, here is my effort.
I decided to go third-world on this one and spend zero money. So, what you see is pretty much an assortment of junk I had lying around. (I figured
such an approach was my best chance and fitted best with the general aim of the comp -- low tech method for producing HNO3 suited to as wide a
spectrum of people as possible.)
The concept was as follows -- assisted in part by deltaH who was thinking along the same lines as me and offered a couple of thoughts upthread.
Combust a high protein grain/bean/pulse/legume at high temperature in excess oxygen in an effort to produce NOx
Trap the NOx exhaust gases by either bubbling through cold water or scrubbing with a fine water spray. The latter is expected to give a better
total yield. The former could result in higher concentration. (Note that H2O2 would be undoubtedly better but this can be as difficult to obtain as
HNO3 in some parts of the world and violated my third-world approach.)
Distil to hopefully produce some HNO3 -- probably still contaminated with a decent amount of dissolved CO2 SO2 and whatever other junk was in
the exhaust gas.
I chose a mix of green lentils (25.8% protein) and peanuts (24.9% protein) for my starting material; reasoning that the peanuts should burn well with
their high oil content and resulting high calorific value. The lentils were my first choice since they were the highest protein grain that I could
find. I figured that they would moderate the fire somewhat and that I would have less CO2 output.
I used a galvanised steel pipe I had floating around as a furnace. It was placed at an angle to chimney the gases through.
I wanted to attempt both the spray method and bubbling through water method. I constructed a spray chamber from a tin can and a 3L juice bottle. It
was not nearly as large as I would have liked but it was the best that I had on hand. Sprayed water came via a garden pump sprayer. PVC hoses were
connected to the spray chamber to collect both the water solution and the gas. The gas was bubbled through a container of ice water (in theory).
The flaws in the design were twofold (as you will readily see if you look at the photos).
1. The furnace design with the chosen fuel did not get hot enough to sustain combustion well. I was prepared to blow air through with a hair drier
or garden blower. This would likely have helped if it were not for the second flaw.
2. The spray chamber was not designed to cope with the volume of exhaust gas that was produced. Therefore it smothered the fire when attached to the
furnace.
At this point, well, ordinary life intervened and I had to dismantle and abandon. If I were to attempt it again I would do the following:
1. Use peanuts only to get a nice roaring blaze.
2. Make the furnace fully vertical and have the peanuts on some kind of grate.
3. Blow air through continuously once the fire was established. Probably not as vigorously as a garden blower, but something.
4. Add a mechanism for adding additional fuel to the system.
5. Feed the exhaust gases into a large drum to act as a spray chamber. Use nozzles that produce a very fine mist. Have a pump circuit to recycle
the water to maximise yield. Add baffles to increase the retention time of the gas in the drum but without restricting the flow too much.
I figured at this point I had stepped beyond a Saturday morning project using available junk. Not beyond the realms of possibility, but beyond what I
could feasibly accomplish with the time I had.
Anyway, here are some pix.
https://www.dropbox.com/s/rpqyw6tnwmwdbdr/2015-01-10%2015.20...
https://www.dropbox.com/s/4c0zplvarqxj2fm/2015-01-18%2014.12...
https://www.dropbox.com/s/b4zjxdcbv5ltr3s/2015-01-18%2014.13...
https://www.dropbox.com/s/f0r70qee0uvy6xk/2015-01-18%2014.14...
https://www.dropbox.com/s/89w4z8oapa7kjbh/2015-01-18%2014.27...
https://www.dropbox.com/s/be3lyjcsta1h5ni/2015-01-18%2014.27...
https://www.dropbox.com/s/lmf9okv0pg5i3pf/2015-01-18%2014.27...
https://www.dropbox.com/s/lhi36ox43gui1bu/2015-01-18%2014.55...
In summary, not the most spectacular attempt and certainly does not meet the brief. But on the other hand, the only actual attempt presented in this
comp so far.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Fuck I thought it ended at the end of the month! Dammit.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Kudo's for your effort j_sum1 and taking the time to present it and your findings. Loved the spray chamber  I think you may have gotten tantalisingly close! I think you may have gotten tantalisingly close!
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Quote: Originally posted by j_sum1  | | I am genuinely surprised to find that the submissions are so... um... nonexistant. There have been a large number of good ideas floated. None
without certain difficulties, but that is the nature of the competition. |
I wouldn't write off ol' WGTR just yet, haha! I've been slaving away, trying different configurations of this and that, probably spending way too
much time working on this. One wouldn't believe how much effort it takes to make something simple and easily duplicable by the masses. If the goal
was to find the most complex solution, I could have done that easily. In the end, a compromise will have to be reached. An easy design will be an
expensive one. An inexpensive design will require more skill from the constructor. I'm not sure that both "cheap" and "easy" are possible at the
same time, especially since both terms are relative to a person's resources and skill. The current design is reaching a point of sufficient
compromise, I think.
I am going to school and working full time, so that has slowed me down quite a bit.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I think that j_sum1's setup is quite clever. The first thing I noticed was how well his collection of random junk bits fit together.
It might sound like I'm joking, but I'm really not.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Woohoo !
I knew somebody would come up with something.
"The Winner will be the one who posts the Best overall solution"
Unless i'm mistaken j_sum1, yours is the Only solution, making you the Winner !
Does anybody disagree ?
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I'd like to point the fact that even if his setup is remarkably well built for rubbish, there is NO WAY on earth it could be used to make any yield
whatsoever by burning beans and peanuts.
Just my two cent
[Edited on 26-1-2015 by plante1999]
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
browsing some older threads about today i got to the idea of reacting urea with ozone, cant say if this would work at all, but it would generate 2
moles NO3 per mole urea and one mole CO2 -- IF it could be done, im not a total wizard when it comes to figuring out whether reactions could take
place
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
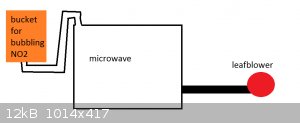
i think j_sum1's method could work better if a microwave were used to heat the material while it's burning
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Must I remind you of the objective.
Objective: Make at least 100ml of 30w% Nitric Acid in the easiest and cheapest way possible.
This isn't over until someone has produced 100ml of 30% w/w of Nitric Acid.
I have a few papers describing the production of dichloramine as the dominant product in solution (never been isolated before), which can then be
oxidized to various products, including nitrogen gas (N2), nitrates (NO3), nitrous oxide (N2O), nitric oxide (NO), and others not mentioned.
This is an existing problem within water purification plants where they have to remove the nitrates being formed (albeit biologically), but it is an
oxidative process, so it should be able to be done synthetically.
I have also been reading into acid rain, of which, HNO3 is a constituent, so I am learning more about that process and to see if it can be scaled up.
[Edited on 26-1-2015 by Loptr]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Well, i did say that i'd take the opinions of others into account ...
j_sum1 is the only entry though, so that kind of narrows it down a bit.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I close my eyes for a bit and wake up in the midst of a controversy. I must admit to being somewhat flattered.
The truth is undoubtedly somewhere between these two sentiments.
I never considered myself a serious contender here but thought it worth a shot. And the only reason I posted anything (after what I thought was a
failure) is because I thought the challenge worthwhile in and of itself. It seemed silly to put the effort in and not show my findings to give others
a leg up.
And plante1999, you are very correct on this. It all comes down to yield – whatever method is employed. All routes were going to be a struggle –
whether one is burning beans, making sparks in the air, babysitting a pile of faeces or attempting to react urea with oxygen (with whatever pressure
could be attained and without a Pt catalyst). I note that even commercially, this last method has ridiculously low yield and most of the feed has to
be diverted back into the system again.
With my embryonic lab I knew that the 100mL 30% was likely out of reach. Hence work on a proof of concept. If HNO3 acid rain occurs naturally then
at least my concept was feasible.
Thanks all for the comments on my junk assembly. I was pleasantly surprised at how well it all fitted together. What you can't tell from the photos
is the thing seals quite well. I am going to keep the spray chamber for – something anyway. Maybe SO2 capture.
The efficiency of my junk assembly was really a shortcoming though. I overlooked a simpler solution that would have accommodated the gas flow much
more easily. If this thread remains open or a new one spawns from it then I will post my idea. I am not done with this concept even if the deadline
is passed.
Quote: Originally posted by Loptr  | Must I remind you of the objective.
Objective: Make at least 100ml of 30w% Nitric Acid in the easiest and cheapest way possible.
This isn't over until someone has produced 100ml of 30% w/w of Nitric Acid. |
Actually, I thought this was over on 24 January.
So, in terms of the comp, it really comes down to how rigorously aga defines "best solution" and "24 Jan". And that would be up to him. I am
intrigued by the late ideas and hints at late submissions.
In terms of the challenge of making HNO3, I have learned a lot through reading this thread and researching ideas. (French method? Who knew?) There
are still unexplored avenues here and I feel the challenge is an excellent one on its own merits.
So, back to you aga. It's been fun.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
From page 1 :-
"If January is too short a time, then there need be no Closing date.
If there are No entries before January, the challenge will stay open until the beer runs out, which doesn't look like anytime soon."
There is still Beer here, despite my best efforts, and j_sum1 seems to be onto something interesting, so we keep it going !
Edit:
Technically nobody Won per se, however j_sum1's approach is wonderful, and deserves at least some banana.
P.M me your paypal address please.
[Edited on 27-1-2015 by aga]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Game on. Let's see what else comes up. I unfortunately won't be able to play for maybe three weeks. I am going to mull on my modifications for a
while and see what transpires.
aga. PM coming your way.
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
You guys are one giant bunch of goof balls. I say this with love in my heart, and a smile on my face.
This thread is wonderful, and Mr. Aga... I commend you on your humanity.
Mr. J.sum1... Fantastic! Burning beans. Who would have thunk it. I'm gonna have a look around the house to see what I can spark up.
Edit:
In looking around I did find some common Wallgreens ingredients to produce Nitric acid using simple gas generators or more complex distillation
equipment. They are really nothing more than pour, and play. Too bad they don't fit the rule set. (Ice Pack/Hydrogen Peroxide/a few old pennies, and
some hardware store battery acid) Easier than fermented pee for sure.
[Edited on 27-1-2015 by Zombie]
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
To stave off the SM community's stomach pangs of unfulfilled expectations, I humbly present a preview of Project Dead Woodchuck. It's a work in
progress, but hopefully it will prove to be more useful than its namesake.
Attachment: Rotating_Arc.avi (4.8MB)
This file has been downloaded 1186 times
The electrodes are water-cooled by means of a miniature submersible pump. The electrodes may look complicated, but they were made out of water pipe,
tubing, copper wire, solder, and bits of copper sheet. One drill press offered its services, but otherwise only hand tools were used.
To fit the spirit of the competition, the power supply delivers 150V at 3A to the reactor, in series with a 30 ohm resistor. This type of power would
be available by rectifying mains voltage. I noted that there is a pretty strong smell of ozone in the fume hood, but I haven't taken a sample yet to
look for brown gas.
The arc rotates, but not as fast as I want it to. Note how far away the magnet is from the arc. As I bring it closer, the arc gets drawn to the
center of the ring magnet, where it rotates so fast as to be a solid ring of light. This rapid rotation is desirable, as it greatly improves
electrode life. With the current configuration, though, the magnetic field quickly blows out the arc when it is brought this close. The arc voltage
needs to be higher, or the reactor geometry needs to change.
When the arc doesn't rotate fast enough, small travelling hot spots can develop on the electrodes. These are visible as bright strands of light
present in the overall luminous arc. As the arc concentrates at various points on the electrode, it mildly etches the surface, actually making the
problem worse. The arc gets noisier, and rotation begins to slow down. The arc keeps going, though. The video was recorded from a 30 minute long
test.
Before starting the reactor, I do polish both electrodes and gap them carefully. With this kind of treatment the arc rotates smoothly through the air
gap, delivering a solid luminous ring. Looking through welding lenses, the air gap glows bright orange, with slight tinges of green around the
cathode.
Within a few minutes, however, very small hot spots begin developing around the imperfections on the electrodes. Arc rotation slows down, as the arc
tends to linger in these areas a bit longer. Although the air gap still glows, there is more green coloration present. The electrodes themselves are
not overheating, though. The water keeps them cool.
Anyway, I'll probably be working on this for quite a while. If anyone else wants to try their hand at it, feel free to jump in. I'm not doing this
to actually win anything; that's why I don't mind sharing it.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Ok. Colour me impressed.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Quote: Originally posted by WGTR  | To stave off the SM community's stomach pangs of unfulfilled expectations, I humbly present a preview of Project Dead Woodchuck. It's a work in
progress, but hopefully it will prove to be more useful than its namesake.
|
Now, THATS something that has serious potential..
Good luck!
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by plante1999  | Quote: Originally posted by WGTR  | To stave off the SM community's stomach pangs of unfulfilled expectations, I humbly present a preview of Project Dead Woodchuck. It's a work in
progress, but hopefully it will prove to be more useful than its namesake.
|
Now, THATS something that has serious potential..
Good luck! |
are you kidding ? that's freakin awesome ,tooooo goood
and WGTR says he is in school lol
I think he deserves the prize for just making a thing like that
also,for the burning beans idea ,instead of burning beans, could you mix melamine with some oxidising agent(bleach,permanganate ? ) and then burn
that ?
http://en.wikipedia.org/wiki/Melamine
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
Really guys. I get the outside the box thinking but how does any of this make it easy for joe blow to make nitric acid at home. I thought that was the
point?
I posted 2-3 posts up that I found the stuff right in the house, and every corner store on the planet has the same stuff.
This is turning into a Rube Goldberg competition... It seems contrary to the original intent. BUT it's your game.

They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I think the point is that some items are not available at every corner store. There are many places whee nitrates are restricted. Sulfuric acid is
almost impossible to find otc where I live. And there are historically numerous novel routes to nitric acid.
Therefore to have a highly accessible method for nitric acid is a good idea. Something that is not dependent on certain supplies is also a good idea.
I am not really expecting an armageddon or totalitarian state situation personally but the chemistry implications of such a situation are an
interesting exploration.
|
|
|
| Pages:
1
..
3
4
5
6
7
..
22 |