| Pages:
1
..
3
4
5 |
Azmat09
Harmless

Posts: 2
Registered: 29-12-2009
Member Is Offline
Mood: No Mood
|
|
hello;
i want to know that cant we generate NH3 and HCl by the electrolysis of NH4Cl ,or by any other mean
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
What does this topic have anything to do with producing NH3 and HCl from NH4Cl? This is about perchlorate acid preparation - HClO4.
|
|
|
Azmat09
Harmless

Posts: 2
Registered: 29-12-2009
Member Is Offline
Mood: No Mood
|
|
sorry im new here .i didnt knw where to put my question
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
When NH4Cl is electrolysed, hydrogen is evolved to first produce monochloramine, then dichloramine and finally nitrogen trichloride.
All three compounds are toxic and the trichloride, an orange-coloured, oil is dangerously explosive.
|
|
|
Shotgun Man
Harmless

Posts: 4
Registered: 10-12-2011
Location: The Nanny State
Member Is Offline
Mood: Mood Status Pending
|
|
Perchloric Acid from ClO2
Some input here,
Assuming that Plasma and Madscientist were correct in stating that ClO2 in hot water will generate HClO4, but taking into account Theoretic mentioning
you require UV to form HCl and HClO4, as well as the success of JoPann using this method via the use of sunlight and predissolved relevant substances
for the formation of HCl and HClO4...*gasps for a breath*... I have an idea that I want to put past you guys. Feel free to try it as it will take me a
while to get around to it and post any notes on it. Also understand you do this at your own risk and I am not liable for any damages incurred by you
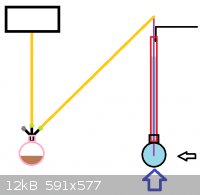
crazy bastards... The attachment contains a simple image using MSpaint to explain the set up.
First a little theory... according to some fast google searches and some youtube video demonstrations involving ClO2 decomposition, it seems that it
is only handled dissolved in liquid for industry. This is due to the fact that decomposition at 10% by volume to air volume and up can occur. This
would have lead to plasma's unfortunate lab accident. The solution would be to handle very dilute amounts of ClO2. Also supposedly there is a
requirement for a UV source. UVA, UVB, UVC I am not certain about which will maximise yield, however glass will block large amounts of UVB and UVC so
will require the source to irradiate the water directly. UVA at longer wavelengths can pass through glass and is conveniently sourced through sunlight
in relatively large amounts. The success of JoPann in his experiment shows the method is possible and would benefit from a more continuous system.
Onto the image, this is my crude set up for proof of concept...I understand the safety issues and design flaws, but I do not conduct experiments
indoors or in a city like you lunatics.
-Black rectangle = aquarium air pump
-Orange line = appropriate chemical resistant tubing (PVC?)
-Green Dot = stoppers with method to accommodate tube (airtight)
-Grey Dot = stopper
-Black Tripod on Pink Flask = 3 neck adapter
-Pink Flask = mixture (Brown) to generate ClO2
-Black Flask = filled with water
-Red Tube = 400mm distillation apparatus used to extend scrubbing time
-Purple Line = glass tubing
-Black Line = UVB/UVC source
-Black Arrow = UVA
-Purple Arrow = Heat source
The idea of this system is to force large volumes of cool air through the reaction vessel to prevent a 10% ClO2/air scenario, while allowing
irradiation of the water simultaneously as the gasses are dissolved.
This system is inefficient and a serious chemist would create scrubbing towers filled with glass media with Geyser pump and ample scrubbing time with
irradiation points located at various points and great atmospheric ventilation. However, the purpose was to demonstrate most importantly safety via
elimination of ClO2 decomposition and the rate and efficiency of UV conversion from dissolved gasses to desired products. If pathetic yields of HClO4
are detected from a single run (possibly 30 minutes to 2 hours) of the system, it will be a success as the only problem left is designing cost
effective and efficiency scrubbing systems.
If anyone has any dream crushing theory as to why this system is useless then definitely say something as I suspect this method is far too simple to
elude industrial use. However at the same time I feel it may be too costly for industry, much like how the birkeland-eyde process is feasible but too
costly, and so may be a viable route for the amateur chemist should it be a success. Thanks chaps!
"While all other sciences have advanced, that of government is at a standstill - little better understood, little better practiced now than three or
four thousand years ago." - John Adams
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Here are some of my thoughts/references from a recent thread (link: https://www.sciencemadness.org/whisper/viewthread.php?tid=40... ) addressing the action of UV light on gases ClO2 as a path to dry perchlorates
that may be of interest in your related proposed treatment of ClO2 in hot water and/or path to HClO4.
Quote: Originally posted by AJKOER  | Here is a new idea to prepare dry perchlorates, which is untested and requires some feedback, but otherwise may seemingly be doable. First prepare
Chlorine perchlorate, a pale greenish liquid which decomposes at room temperature, formula Cl2O4 or better ClOClO3. It is produced by the photolysis
of chlorine dioxide at room temperature with 436 nm of ultraviolet light:
2 ClO2 → ClOClO3
Source: See Wiki and references therein, link: http://en.wikipedia.org/wiki/Chlorine_perchlorate
Also, see "Chlorine Perchlorate Formation in the Gas Phase Photolysis of Chlorine Dioxide" by F. Zabel, link: http://onlinelibrary.wiley.com/doi/10.1002/bbpc.19910950809/...
"Abstract
OClO/O2/N2 mixtures were photolyzed in a temperature controlled 4201 reaction chamber at temperatures between 249 and 300 K and total pressures
between 0.5 and 1000 mbar. Initial OClO concentrations were in the range (1.7–5.7) · 10^15 molecule/cm3. Reaction mixtures were analyzed in situ
via long-path IR absorption using a Fourier-transform spectrometer. In some experiments product spectra were simultaneously monitored in the IR and
the UV. Depending on reaction conditions, the product IR spectra were dominated by absorption bands of Cl2O3 or Cl2O4 or a mixture of both. Evidence
is presented for the crucial role of O atoms in the Cl2O4 formation, suggesting either of the two mechanisms: (I) OClO + O + M → ClO3 + M
→ ClO3 + ClO + M → Cl2O4 + M, or (II) OClO + ClO + M → Cl2O3 + M, Cl2O3 + O + M → Cl2O4 + M. Both the weak temperature
dependence and the strong pressure dependence of the Cl2O4 yield support mechanism (I). In addition, Cl2O6 was detected as a minor product of OClO
photolysis under certain reaction conditions, both by its IR and UV absorption."
See also "Novel ultraviolet product spectra in the photolysis of chlorine dioxide", link: http://pubs.rsc.org/en/content/articlelanding/1984/f1/f19848...
"Abstract
U.v. absorption spectra have been recorded during the low-intensity photolysis of chlorine dioxide, OClO, using a diode-array spectrometer. A
broad-band u.v. spectrum was observed which was favoured by low temperature and high OClO pressure. The absorption could be explained only in part by
the presence of ClO dimer, Cl2O2. Unequivocal assignment of the residual spectrum was not possible but it may be due to the chlorine perchlorate
molecule, ClOClO3, a recently discovered product of OClO photolysis."
Now, per Wiki Chlorine Perchlorate "is less stable than ClO2 and decomposes to O2, Cl2 and Cl2O6 at room temperature.
2 ClOClO3 → O2 + Cl2 + Cl2O6
Chlorine perchlorate reacts with metal chlorides forming anhydrous perchlorates:
CrO2Cl2 + 2 ClOClO3 → 2 Cl2 + CrO2(ClO4)2
TiCl4 + 4 ClOClO3 → 4 Cl2 + Ti(ClO4)4 "
which is a possible new path to the current thread topic of the production of perchlorates.
With respect to handling Chlorine dioxide per Wiki: "At gas phase concentrations greater than 30% volume in air at STP (more correctly: at partial
pressures above 10 kPa [7]), ClO2 may explosively decompose into chlorine and oxygen. The decomposition can be initiated by, for example, light, hot
spots, chemical reaction, or pressure shock. Thus, chlorine dioxide gas is never handled in concentrated form, but is almost always handled as a
dissolved gas in water in a concentration range of 0.5 to 10 grams per liter."
So having the means of producing the correct frequency of UV light to foster the reaction, noting the reaction temperatures, pressure and ClO2/O2/N2
inert gas concentration mentioned above (limiting the explosion hazard), may indeed provide a new path to dry perchlorate production. EDIT: The
following article states at room temperature that Cl2O4 is the major product of the photolysis of ClO2 with both a continuous wave (mercury lamp) and
pulsed (XeCl UV laser) light sources. Link: http://pubs.acs.org/doi/abs/10.1021/j100221a001
I also think it would be interesting in trying to dissolve ClO2 in an organic solvent (like CCl4) to form a dilute solution (under 15%), as per one
source (http://www.thesabrecompanies.com/science/chemistry.aspx ) ClO2 is highly soluble in solvents and oils as in water. Then, treat with the proper UV
exposure to create Cl2O4 and then add a suspension of say, dry CrO2Cl2, to create a perchlorate salt in a stainless steel vesel. My reading of
associated patents (see Patent 4012492 on "Synthesis of anhydrous metal perchlorates") of employing Chlorine Perchlorate in forming perchlorate salts
is that current known salts can be produced although with some new perchlorates (titanium tetraperchlorate, vanadium perchlorate, and
chromylperchlorate) can so be directly formed (link: http://www.google.com/patents/US4012492 ). |
Also, the comments:
Quote: Originally posted by AJKOER  | As this thread involves the discussion of perchlorates, I can across a possibly valuable source. See https://sites.google.com/site/energeticscribble/perchloric-a...
On precautions involving HClO4, please note the author's comments. In particular, the author notes that "For CCl4, HClO4 is insoluble in CCl4, and
gives upon shaking, a green emulsion, which discolors brown after several minutes welling up under formation of HCl and COCl2 (Vorländer, v.
Schilling, Lieb. Ann. 310 [1900] 374)" and further "So mixing something like CCl4 and HClO4 can cost one their lives if not wearing protective gear,
doing under fume hood,etc. It is dangerous to extrapolate so assuredly."
|
Also, with respect to electrochemical path to HClO4, I have previously noted that in the case of chloride free HOCl (dissolving Cl2O in water is one
preparation), the disproportionation reaction of HOCl proceeds to perchloric electrochemically. To quote "concentrated Cl-free HOCl can be oxidized
electrochemically to chloric and perchloric acids (97)." Page 554. The reference (97) is a patented process by World Pat. 9,114,614 (Oct. 17, 1991),
D. W. Crawford and co-workers (to Olin Corp.). See reference at "DICHLORINE MONOXIDE, HOCl, HYPOCHLORITES", Volume 8. Link: http://www.scribd.com/doc/30121142/Dichlorine-Monoxide-Hypoc...
With respect to the reaction of hot water, ClO2 and UV light, I would expect the creation of Cl2O4 and its decomposition (as noted above) into O2, Cl2
and Cl2O6 (or, structurally O2Cl-OClO3), the latter reacting with water as follows:
Cl2O6 + H2O --> HClO3 + HClO4
See: "Inorganic Chemistry For Undergraduates" by Gopalan, R., page 511.
Link:
http://books.google.com/books?id=Fs4zQ-hNTz8C&pg=PA511&a...
Per another source, I would expect the hydrolysis of Cl2O4 in cold water to proceed as follows:
Cl2O4 + H2O --> HOCl + HClO4
or, structurally:
Cl-OClO3 + HOH --> HO-Cl + H-OClO3
See Figure 1 in "Chlorine Oxoacids and Structure of Dichlorine Oxides", page 277.
Link: http://mdp.academia.edu/SandraQuiroga/Papers/1621127/Chlorin...
[Edited on 24-7-2012 by AJKOER]
|
|
|
| Pages:
1
..
3
4
5 |
|