| Pages:
1
..
44
45
46
47
48
..
60 |
learningChem
Hazard to Others
  
Posts: 182
Registered: 21-7-2011
Member Is Offline
Mood: No Mood
|
|
some notes...
I started with (NaPO3)6 and did
a) (NaPO3)6 + 3CaCl2 --> Ca3(PO3)6 + 6NaCl
b) Ca3(PO3)6 + 3H2SO4 --> 3CaSO4 + 6HPO3
Ca3(PO3)6 is a funny substance. It's a sticky mess. Doesn't look like an ordinary salt at all (I guess these P compoounds are some kind of polymer?)
I concentrated the HPO3 solution by heating. When it cooled down, I got a mass of crystals. See picture.
In theory that stuff is rather pure HPO3, but I'm not 100% sure.
The color of the retort was kind of orange, but not too bright.
ElementCollector
| Quote: |
because you didn't use vacuum or inert gas, your yield is likely much lower than that. |
Well, apart from P, the products of the reaction are H2 and CO. Wouldn't those gases displace any O2 present? Also, I don't think there was any P2O5
to be seen.
If I recall correctly there was a fair amount of C left in the retort. Or at least a black slag, which should indicate that there was too much C or
too little HPO3?
Anyway, I don't completely trust the starting materials, and I was more interested in finding out whether some kind of reaction would happen at all
using my furnace.
I'll do a more careful test. Also, I might buy some phosphoric acid.

[Edited on 8-12-2013 by learningChem]
|
|
|
elementcollector1
International Hazard
    
Posts: 2689
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
True that. Unfortunately, there was already air in the tube before the reaction, so it's not out of the question. Also, the hydrogen and carbon
monoxide would have diffused into the air in the tube, so you'd likely get an unholy mix of the three.
I'm assuming you didn't distill off the phosphoric acid, which is normally clear crystals. Try a recrystallization if you're not sure about the
purity.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
bfesser
|
Threads Merged
5-1-2014 at 07:49 |
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
I found some papers (written by me), where I noted several experiments that I made in the past summer (or I intend to make).
I think that...these informations will be helpful:
  
Attachment: converted_to_.pdf (1020kB)
This file has been downloaded 1442 times
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: PDF]
[Edited on 30.1.14 by bfesser]
|
|
|
bfesser
|
Thread Split
30-1-2014 at 12:37 |
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
I think I forgot to tell you something  : :
All operations involving white phosphorus should be conducted in the absence of light (especially short wavelength light) because white phosphorus can
turn into red phosphorus, which is much less reactive.
...P4 molecule is freaking annoying 
[Edited on 31-1-2014 by DoctorZET]
|
|
|
PeeWee2000
Hazard to Self
 
Posts: 58
Registered: 2-7-2013
Location: Michigan
Member Is Offline
Mood: No Mood
|
|
Thats a pretty spiffy looking setup idea there ZET, carbon disulfide seems kind of exotic to me though is it necessary for capturing the phosphorus or
could you eliminate it and try to just condense the phosphorus in cold water if you're willing to live with some impurities?
I've been wanting to be able to get ahold of phosphorus for some time but haven't bothered with all the attention it gets from the DEA and lack of
need that I have for it but this method would be perfect for me if its really that simple!
“Everything is relative in this world, where change alone endures.”
― Leon Trotsky
|
|
|
elementcollector1
International Hazard
    
Posts: 2689
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Problem is, that conversion to RP is actually very slow - only the surface is converted, if I recall. So, light is fine.
Doc ZET, is this an experiment you did or one you plan to do? The theory looks fine from a surface glance.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
Hmmm...I think it should also work with carbon tetrachloride/chloroform/benzene/toluene (this solvents can easily dissolve white phosphorus) but be
careful with first two solvents (because, phosgene can be formed in the CO+H2 output flame).
Also you can try to collect P4 vapors in cold water, then boil the water and release the white waxy phosphorus sample into the flask. I'm not sure
that it will work , unless I don't try this method yet. (there can be traces of H3PO4 and other phosphorous acids in water, but not in CS2 or other
nonpolar solvents)
And yes, I did this experiment (on a small scale). I have got only 2-3 grams of phosphorus ... but my goal was not to obtain phosphorus.
Guess who was my source of phosphoric acid? >>> Answer is seven bottles of Coca-Cola. 
First, I boiled all of the juice for 2 minutes (to destroy the carbonic acid), then added 30 grams of Ca (OH) 2 to precipitate calcium phosphate.
Then I added calcium phosphate in concentrated sulfuric acid, I distilled the mixture and voila! A few grams of almost pure phosphoric acid from Cola!
(almost dry).
My goal was to determine experimentally how much phosphorus is in Coca-Cola. 
To be sure, use acetylene instead of ethylene, but be careful! Acetylene can ignite/explode in the presence of an oxygen source (like NO2, N2O3, CuO,
SnO2, PbO, H2O2 ,ClO2 ,HClO2 and, why not H3PO4 ) at that temperatures (600*C).
[Edited on 31-1-2014 by DoctorZET]
|
|
|
elementcollector1
International Hazard
    
Posts: 2689
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Interesting! What did you find in terms of yield of phosphoric acid from x bottles of Coke? (example, how many grams of
Ca3(PO4)2)
I've had problems with sodium orthophosphate and sulfuric acid - mainly due to contamination with carbonates (calcium would not be an effective
separating agent here...)
How did you add the Ca(OH)2? In solution, or solid? If solid, how did you separate this from the Ca3(PO4)2
- just by mixing stoichiometrically, or was there something else?
This sounds like a potentially more viable procedure than the usual high-temperature stuff, mainly because the reagents are (relatively) easy to find
or synthesize, and the procedure itself looks sound.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
As I said (in the pages), the reaction between ethene and phosphoric acid at 600 * C in the the presence of CuO is exothermic, and if you try to scale
up to much, you can get it out of control and produce phosphine, a toxic gas (smells like garlic when burned).
About the phosphoric acid in Cola...I don't remember the exact concentration...but I remember that if you add enough Ca(OH)2 in the boiled juice, the
solution will turn basic and the calcium phosphate will start to decant at the bottom of the pot (along with the excess of Ca(OH)2 ).
Then collect the CaHPO4+Ca3(PO4)2+Ca(OH)2, remove the water from the mixture by heating it, and add the mixture to some fuming sulphuric acid (not to
much, but a little excess).
After it reacts, you can distill almost pure phosphoric acid.
(CaHPO4 represent actualy some hidroxyapatite formed in the acid water solution, while the H3PO4 reacts with Ca(OH)2 )
[Edited on 31-1-2014 by DoctorZET]
|
|
|
PeeWee2000
Hazard to Self
 
Posts: 58
Registered: 2-7-2013
Location: Michigan
Member Is Offline
Mood: No Mood
|
|
Awesome now I have a reason to get an acetylene tank! Now my welding supply company wont wonder why I only have an oxygen tank  And toluene is definitely the most convenient for me, I can buy it by the gallon no
problem. Cant wait to try this out! And toluene is definitely the most convenient for me, I can buy it by the gallon no
problem. Cant wait to try this out!
I sure wish I had the patience and determination to want to figure out the phosphorus content of a bottle of coca cola, props for that ZET! Hope I get
to see some more of those awesome sketches elsewhere on the forum too!
[Edited on 31-1-2014 by PeeWee2000]
“Everything is relative in this world, where change alone endures.”
― Leon Trotsky
|
|
|
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
Thanks...but (just asking) ...you have read the section in which I said:
"Acetylene can ignite/explode in the presence of an oxygen source (like NO2, N2O3, CuO, SnO2, PbO, H2O2 ,ClO2 ,HClO2 and, why not H3PO4 ) at that
temperatures (600*C)."
and, I also say that :
" the reaction between ethene and phosphoric acid at 600 * C in the the presence of CuO is exothermic, and if you try to scale up to much, you can get
it out of control and produce phosphine, a toxic gas (smells like garlic when burned). "
so...using an acetylene tank for these procedure...it's a "little" bit dangerous...I think...(because you work with phosphorus, and with a lot of
flames around, and with a HUGE source of acetylene, and with a 2-3 meters hot red copper tube in which some dangerous&exothermic reduction
reactions take place)
[Edited on 31-1-2014 by DoctorZET]
[Edited on 31-1-2014 by DoctorZET]
|
|
|
DoctorZET
Harmless

Posts: 42
Registered: 25-1-2014
Location: In the lab
Member Is Offline
Mood: tasting a pure sample of madness
|
|
better use calcium carbide 
and there is another something:
3CuO+H3PO4+2C2H2 =(550*C)=> Cu3PO3+4CO+(7/2)H2+ [ KABOOM ],
where the 'kaboom' is the main problem...
then, the heat produced by first reaction activate this reaction:
Cu3PO3 <=(630-700*C)=> 3CuO+ P^
and the CuO is reintroduced in the process
[Edited on 31-1-2014 by DoctorZET]
[Edited on 31-1-2014 by DoctorZET]
|
|
|
PeeWee2000
Hazard to Self
 
Posts: 58
Registered: 2-7-2013
Location: Michigan
Member Is Offline
Mood: No Mood
|
|
Yeah It might not have all been fresh in my head in that last post though  Luckily
I'm only fantasizing right now, usually I'm pretty through with my safety though. And besides I dont actually have the money to buy an
acetylene tank for a single reaction Luckily
I'm only fantasizing right now, usually I'm pretty through with my safety though. And besides I dont actually have the money to buy an
acetylene tank for a single reaction 
“Everything is relative in this world, where change alone endures.”
― Leon Trotsky
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Ok I spent 12 hours reading the topic and made some 11 pages of notes from what people were doing and thinking around there.
I noted that nobody didn't consider the Wöhler process of using electric furnace for this reaction. It certainly gives enough heat. My worry is that
will the reaction mixture be conductive enough to allow current pass through. My idea is to make a tubular reactor as following:
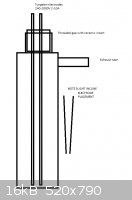
The reactor volume would be 1-2L. To there, two tungsten electrodes with ceramic steel lined threaded plug is inserted according to picture. Also
carbon rods could be used, but I've got some tungsten floating around. I would wanna test using direct microwave transformer pushing 1kW at 2000V to
induce a gap width of about 10-20mm for electric arc. This way the arc wouldn't be dependent on the mixture's conductivity, but if it turns out it is
well conductive once started, a voltage of 20-30V with high amperage(in scale of several dozens) should be used.
The electrodes should be inclined so the arc will initate at the bottom. A ballast of heating elements would be placed in the circuit so the fuse wont
short out and the reaction energy is limited by the respectiveness of the mixture's temperature. The circuit would be fitted with power adjuster. The
body of the vessel is stainless steel so it could become conductive and therefore require insulative placement.
Several reaction mixtures should be tested. First is the classic mixture of 10Al + 6 NaPO3 + 3 SiO2 ball milled. Another mixture consists of Ca3(PO4)2
+ 3 SiO2 + 5 C. The heat from the arc should be more than sufficient to squeeze out what we want. No inert gas is required since at the moment of
initiation, the small volume of the reactor is occupied by millimoles of reactive atmospheric gases and therefore burnt to oxides in the next second.
On the side would be the exhaust port where a steel tube is lead underneath water heated to 50-60C.
Another option consists the same reactor structure, but the heating is applied through the reactor wall, as in electric furnace or by coke bed. Steel
merely holds up to 1200-1400C before becoming too soft and reactive, so it could be replaced with Al2O3+silicate or MgO refractory with proper
fittings.
I'm interested in the practical scalability of this reaction, eg. could this produce WP in scale of several moles per run. In the posts regards
towards too finely mixed reactants caused explosive initiation when the batch was scaled up slightly, which must be taken into consideration until
bringing it up to several hundreds of grams, or even kilograms. The cost of the reaction is of another matter: if the reactants of Al and B could be
substituted with carbon and SiO2, the cost and the trouble of making the reactants will decrease substantially. With the most expensive setup with
fine Al and Boron, the cost and the trouble of making the reactants themselves can become a major limiting factor.
[Edited on 9-2-2014 by testimento]
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
"Ok I spent 12 hours reading the topic and made some 11 pages of notes from what people were doing and thinking around there "
Great, you should put them online as a synopsis of a rather long winded thread.
|
|
|
elementcollector1
International Hazard
    
Posts: 2689
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
That's kind of already been done?
http://www.sciencemadness.org/talk/files.php?pid=247779&...
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
I stand corrected!
|
|
|
thebean
Hazard to Others
  
Posts: 116
Registered: 26-9-2013
Location: Minnesota
Member Is Offline
Mood: Deprotonated
|
|
I had an idea while I was reading this thread, but it sounds insane. I"ll propose it anyways.
3 P4S10 + 20 Al -> 3 P4 + 10 Al2S3
My plan would entail loading the P4S10 and Al into a retort made of steel pipe or maybe borosilicate glass. Then I would heat the mix with the end of
the retort in water. Maybe a different reducing agent would work better.
"You need a little bit of insanity to do great things."
-Henry Rollins
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Not necessarily. Have you at least checked whether the reaction is thermodynamically favourable?
|
|
|
Litmus
Harmless

Posts: 3
Registered: 28-2-2014
Member Is Offline
Mood: mad
|
|
Ok Perhaps I'm just a dumb-ass. I've been following op on this +decade long thread as best I can. So far I've caught up to about 2010 or so so
please understand me if I'm not up to speed on the recent posts. I eventually shall, but with such a phase lag involved it'll be a little while for
me more to catch up. Perhaps a spin-off thread of sorts would be in order. I'm new here, so please fill me in for any my missing spaces as a
newbie. So far the German garage chemist, blogfast, peach, Wack, and Roger have contributed so much in a "thread" that was pioneered more or less
by Polverone and Bromic Acid long ago. These guys have poured heart and soul into making this fascinating substance, Phosphorus. which happens to
be an element. Rather amazing how far mere scientific curiouity and perseverence will go. I've heard it before that the chemists were ahead of the
physicists at the time in terms of new discoveries. Long Before the periodic table was discovered, chemists were at it discovering all sorts of
things by mere good "detective work" .
About time there was a forum for chemistry like there's been for the Linux/GNU/BSD crowd forever.
Okay the facts from literature I know about Phosphorus:
Quote: from my old CRC Handbook of Chemistry and Physics, 62nd Ed 1981-1982
at. wt. 30.97376; at. no. 15; m.p.(white) 44.1 C, b.p. 280 C; sp. gr. (white) 1.82, (red) 2.20, (black) 2.25-2.69.
White P has twp modifications: alpha and beta with a transition temp of -3.8 C.
Discovered in 1669 by Brand, who prepared it from urine.
Never found free in nature, it is widely distributed in minerals.
Phosphate rock, which contains the mineral apatite, an impure tri-calcium phosphate, is an important source for the element.
Large deposits are found in U.S.S.R., in Morrocco, and in Florida, Tennessee, Utah, Idaho
etc, etc. Insoluble in water...
Yes, insoluble in ideal water, which contains no dissolved gases. Real water: O2(albeit slightly), and CO2(a lot).
An old edition of CRC, but doubt a whole much has changed...
Here's a naive thought to throw out to the crowd:
For a long time I've been reading posts about how this annoying obnoxious byproduct called phosphine is often obtained by all the prodigious attempts
I've been reading here to make elemental phosphorus from crucibles at 500-100o degrees C. The thought comes to mind: can you make lemon-aid out of
lemons somehow by reaction of phosphine, where P is in it's LOWEST oxidation state(P-III). and just oxidize that(perhaps in-situ somehow so you don't
pass out by the fumes). with an alkene or alkyne, the simplest being ethylene or acetylene, respectively. If you get the stoichiometry and heat of
reaction right, ought to get the stuff in gobs, right? Or...
Interesting that is NH3 so similar in ways on the PT dissolves in water easily while PH3 don't. If you thought PH3 is bad,
Arsine is insane: even more lethal, as gaseous, and less soluble than phosphine.
In any event, makes common sense the white form is waxy and less stable than the red form.
The P4 molecules are unlinked in white while they're somewhat polymerized in red. Question is, why does
that give it a red appearance and other difering properties such as solubility in Carbon Disulfide(if you're an organic
chemist, just about anything is soluble in CS2, but may not care since the stuff is so gross)
Perhaps it could be incorporated into some gift candle for some irritating relative 
Could the paraffin "stabilize" the P like in the movies' pyro stuff 
George A Bell
|
|
|
rcfunker
Harmless

Posts: 5
Registered: 23-3-2014
Member Is Offline
Mood: No Mood
|
|
Thank you for sharing the link, looking forward to read it!
Quote: Originally posted by Polverone  | | Wow, don't everybody try to answer at once. Anyway, I've just found a very interesting document: US Patent 6207024 (accessible through www.uspto.gov) describes the production of elemental phosphorus from pyrolytic carbon and phosphoric acid, using microwaves to heat the reactants.
The reaction takes place at much lower temperatures than the conventional arc furnace process. The problem (or problems), of course, is that the
phosphorus still needs to be protected from oxidation, you need a relatively heat-resistant and microwave-transparent reaction vessel, and it's going
to be tough to condense and collect the phosphorus if you're trying to come up with something using a domestic microwave oven. Microwave ovens are
cheap at thrift stores, but there are still obstacles to overcome for performing this reaction at home... |
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Would elemental phosphorous float or sink in Liquid aluminium (i.e. molten) ?
If it sinks, the molten aluminium will protect it from Air as it cools ...
https://www.youtube.com/watch?v=0uhI0NwlnL4
[Edited on 11-5-2014 by aga]
|
|
|
Metacelsus
International Hazard
    
Posts: 2543
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
It will react to form aluminum phosphide.
https://en.wikipedia.org/wiki/Aluminium_phosphide
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
<s>float</s>
<s>sink</s>
<b>react</b>
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Are you sure that that is correct, bromic acid?
The delta H of AlP is negative.
When we are talking about the reaction with LIQUID aluminum, the delta S is certainly negative, because we have a liquid turning into a solid
(m.p.=2530 C)
If both are negative, doesn't that mean that the enthalpy only drives the reaction, and the reaction is only favorable at low temperatures?
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
| Pages:
1
..
44
45
46
47
48
..
60 |