| Pages:
1
..
44
45
46
47 |
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
French seller, reliable Beta PbO2 anode but dont run it like that 1 guy who went from chloride to perchlorate.
he makes it himself in his garage using hot lead nitrate plating bath and sells to people.
copper plate the contacts by NaF etching in reverse polarity before plating copper because if you dont copper plate then you wont be able to push
decent current without the contacts glowing red hot.
those electrodes measure 60mm*150mm.
Given perchlorate cells must operate from 200ma/cm^2 to 215ma/cm^2 if we take 200ma/cm^2 the minimum current to operate these electrodes is 36 amps to
38.7 amps.
so unless you have a supply capable of pumping out 36 amps atleast and a cooling setup to prevent overheating of electrodes like water cooled bath
then use smaller electrodes.
Also do this in the winter before of the aforementioned problems with cooling.
use meanwell LRS 350 5 to drive the cell and use 1.5 to 1.8cm distance of the electrodes.
use 2 Ti cathodes with all 3 electrodes copper plated and operating voltage of 4.5v to 5.5v.
This is about what it takes to run these electrodes.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
I have strong air cooling for cells. With the thermostat (sensor on the glass) set to 55 C. The transformer and rectifier also have their own fans.
Why is necessary use the exact current range..? At least 200 - 215 mA/cm2...?
I thought the current might be lower. Process NaClO3 to NaClO4 will take longer. Thats all. I'm in no hurry. With a lower current, for example only
150mA / cm2, the life of the PbO2 surface increases. Electrode spacing 1.5 to 1.8 cm..?. Important, Thanks.
Operating voltage 4.5 V to 5.5 V. Thanks. Isn't that too much?
Thanks ....
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Here is my new 32 amp perchlorate cell!!
https://youtu.be/kW0om52N8SY
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
after making another anode id say the way to guarantee a good plating quality is to have 2 plating solutions and alternating between them.
plate in alpha lead dioxide plating bath as initial layer made from 0.1N lead acetate with 0.4N sodium acetate mixture with exchess Pb(OH)2 or PbCO3
at 15ma/cm^2 initially for 15 minutes to get a strong adherent base coating.
next plate in a suitable beta lead dioxide plating solution containing either lead nitrate, lead chlorate, lead perchlorate with immersed lead metal
for lead perchlorate or slowly fed lead hydroxide to keep the lead ion concentrations up and plate for 2 hours at 6-12 ma/cm^2.
plate alpha lead dioxide again for 45 minutes.
repeat last 2 steps however many times you want until you get the desired thickness around 300 microns.
the beta lead dioxide plating is mostly porous and blue in color and always seems brittle but once you plate some alpha lead dioxide over it turns
shiny and grey and still is very conductive.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Quote: Originally posted by nitro-genes  | Was curious if using isostatic graphite as substrate would allow a good coat of LD to form from a more impure lead nitrate bath. An identical
isostatic graphite rod was plated excactly as posted 2 posts above, using same setup and conditions. First 8 hours of plating at 30-40 mA (3-4
mA/cm2), then 1.5 hours of plating at 600 mA (50 mA/cm2), and finally, 6 hours of plating at 60 mA (4 mA/cm2). Only differences were (1) that both
Cu(II) and Pb(II) concentrations were lower, (2) that the lead nitrate used in this bath was made from fishing weights and (3) the bath had been used
several times for previous plating runs. Very little nitrite was present at the start of the run though.
The result was a visibly good, 1mm thick, coating of lead dioxide. No pinholes or large imperfections. This is probably mainly a function of the
isoelectric and smooth surface properties of the isostatic graphite. Overall the coating seemed very porous though and consisted of small spherical
nodules and/or dendrites loosely held together. When a drop of water was placed on the anode, the water would slowly migrate as through filter paper.
The anode failed after 5 days of use at 2 A (200 mA/cm2) in the cell. The part of the anode above the surface of the electrolyte was completely
intact. The part immersed in the electrolyte completely powdered and eroded away. The LD on the intact part of the anode was extremely well adhered
and could not be separated from the graphite substrate without breaking parts out of the graphite substrate. The LD was recovered as large shards at
the bottom of the cell, without any graphite still attached to it. A cross section of the LD recovered seems to indicate that the porous nature of the
LD is not limited to the surface only, so overall this seems the most likely cause of failure.
Interesting what causes this effect...The lead nitrate produced from the fishing weights always produced these grainy textures, so it seems the purity
of the lead nitrate is very important for plating. From what I've read a nodular appearance could also be due to break down products of the detergent
used or maybe iron contamination. Just shows that even a visibly good coating of LD is no guarantee, it really needs to be dense and shiny. 
[Edited on 27-6-2020 by nitro-genes] |
Wow you know my PbO2 anodes are from fishing weights and yea it produces a blue gray porous rough coating but thats not biggie I just put inbetween
layers of alpha PbO2 using alkaline lead acetate bath to stabalize and strengthen the layers.
This process still leaves a somewhat porous coating which is why it never seemed to work for graphite but worked fine for plating on MMO or conductive
epoxy composite.
The electrodes made from the 2 plating solutions method though can be run at really high current densities with no issues with the most ive run was at
250ma/cm^2
The pic below is my 3rd run with these electrodes with 0.35mm coating thickness and surprisingly the bath does not contain brown or anything and the
coating is still on there.
The cloudiness is my additive of magnesium sulfate which due to pH increase PPT out some Mg(OH)2 which just sits there and is not an issue.
I use the MgSO4 to control the pH and maybe add some sulfates to reduce errosion from left over chloride in the fresh chlorate feed.

|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
new video on outsourced coating process for electrodes.
https://www.youtube.com/watch?v=h1uYoBZp7TE
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
rn im having a strange issue where after test coating some electrodes I wanted to recoat a piece but the damn PbO2 wont come off even when using as a
cathode at 20 amps in 50% NaOH the base alpha PbO2 is so strong it wont come off!!!
The beta PbO2 flaked off in seconds but alpha PbO2 is a tough shit to remove which is a good thing but not if you wanna re use any test electrodes.
I am literally thinking of just cooking with hot oil it to reduce it into other oxides then dissolve in hot acids.
Edit:
found a safer solution involving hot H2O2 and HCl which should dissolve PbO2 and convert it into PbCl2 safely.
[Edited on 21-7-2021 by mysteriusbhoice]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
new copper plated Ti ready to be plated with alpha PbO2.
Copper plating directly on Ti requires a few really cursed reagents to get it to be adherent but after the fact even a belt sander tooks some time
removing a thicker deposit of copper on a Ti plate.

|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
mysteriusbhoice,
What happened with your lead oxide on lead anodes?
I know they have more problems with flaking but what kind of current were you able to get?
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Quote: Originally posted by macckone  | mysteriusbhoice,
What happened with your lead oxide on lead anodes?
I know they have more problems with flaking but what kind of current were you able to get? |
They are meme teir and the issue is eventual diffusion and failure.
Any crack would lead to formation of lead hydroxide which spalls the oxides off and eventual failure of the anode.
I will however explore some more cursed substrates like copper and stainless steel since those can be directly plated onto with PbO2.
The copper plated Ti can easily handle 250ma/cm^2 and the previous coating was stress tested at 426ma/cm^2 and it completed a full run with some
errosion.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
So the only real problem was the flaking. But sounds like you didn't test to failure on amperage.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
OH it can take the amps but the fact that it fails due to diffusion and eventual attack of the base substrate means its unsuitable.
It eventually turns into a white cloudy mess and stops conducting then falls apart so easily.
There is also no real point in pursuing that further since alpha PbO2 from plumbate on Ti or any other substrate is really good for making chlorates.
Anyway heres an update on the PbO2 on copper coated Ti mesh down below.

[Edited on 5-8-2021 by mysteriusbhoice]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
looks nice
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
After adding a final plating in the Pb(ClO4)2 solution it now looks perfect!! ready for testing.
The Pb(ClO4)2 plating solution is doped with F- via 10ml of 10% HF and several unnamed alcohols surfactants were added.
The next update will be after testing the electrode with a full run from chloride to perchlorate at 250ma/cm^2 as per standard.
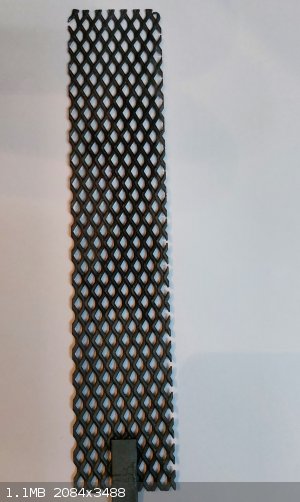
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Ti substrate Cerium-Nickel doped PbO2 before and after coating of PbO2


|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
The errosion rates in chloride liquor of alpha PbO2 vs beta PbO2 is interesting.
Chloride is very corrosive towards PbO2 and will errode it even when doped which is why chloride to perchlorate ends up with muddy brown suspensions.
The beta PbO2 had errosion rates of as much as 30 microns per hour which makes it kind of bad because of this for chloride to perchlorate.
Alpha PbO2 had less errosion at only 2 to 3 microns per hour making it preferred and this is a mesoporous coating and not the nonporous type which is
platedmade at lower current densities.
Alpha PbO2 is overall superior it seems in terms of resistance to chloride electrolyte but its catalytic activity has yet to be tested.
all tests were done at a current density of 200ma/cm^2
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
cerium nanocomposite alpha PbO2 anode.

update:
The errosion rate after 1 hour was 1 micron then next hour less than a micron and the cell liquor changed to slight darker color.
The electrolyte for wear testing is KCl with pH control and current density is 200ma/cm^2.
PbO2 is erroded by chlorides and so far the previous anodes had measurable errosion rates but this one seems to be within the margin of error of the
measuring scale.
I dub thee PbO2 nanocomposite metal oxide (NMO) electrodes
[Edited on 31-10-2021 by mysteriusbhoice]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
ok its official guys.
Alpha PbO2 is superior to beta PbO2 (if plated correctly) the downside is the correct way to obtain a non porous alpha PbO2 layer makes the plating
process AIDS as it takes FOREVER.
about 4x as long as making a beta PbO2 electrode but with its 30x lower errosion rates and more catalytic activity its far superior to beta PbO2.
Essentially beta PbO2 is less active in making perchlorates than alpha PbO2 either that or the alpha plating process is less sensetive to impurities
that can lower its catalytic activity.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Could you elaborate? You say, that the alpha PbO2 layer must be done correctly to be resistant.
So I guess that you put it in 0,1N Pb acetate , 0,4 N sodium acetate solution with lead carbonate as a source of lead ions (won't the lead acetate be
enough?) and then put very low current at the copper-coated titanium strip to obtain the PbO2 layer as non porous as possible right?
What would be the current and exact procedure?
[Edited on 7-3-2022 by mackolol]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Quote: Originally posted by mackolol  | Could you elaborate? You say, that the alpha PbO2 layer must be done correctly to be resistant.
So I guess that you put it in 0,1N Pb acetate , 0,4 N sodium acetate solution with lead carbonate as a source of lead ions (won't the lead acetate be
enough?) and then put very low current at the copper-coated titanium strip to obtain the PbO2 layer as non porous as possible right?
What would be the current and exact procedure?
[Edited on 7-3-2022 by mackolol] |
you need to use 4 molar NaOH and litharge 40g/l heated to 40C using half inserted aquarium heater and tank circulation with stirrer/aquarium pump
placed inside the plating bath.
[Edited on 7-3-2022 by mysteriusbhoice]
Attachment: PGMLess Electrodes 2.0.pdf (12kB)
This file has been downloaded 428 times
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Quote: Originally posted by mysteriusbhoice  | after making another anode id say the way to guarantee a good plating quality is to have 2 plating solutions and alternating between them.
plate in alpha lead dioxide plating bath as initial layer made from 0.1N lead acetate with 0.4N sodium acetate mixture with exchess Pb(OH)2 or PbCO3
at 15ma/cm^2 initially for 15 minutes to get a strong adherent base coating.
|
And this procedure? Does it work? Probably it's worse since you posted the other one.
Never heard of the acetate one, but it seems interesting. Doesn't look like the lead comes from anion though.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
this is an older plating proceedure which worked well enough but the alkaline plumbate gave better results. I tried several plating methods and funny
enough the current density is important.
It must be high for the initial coating and must be dialed down to around 5ma/cm^2 for a while to get a thin but good layer after that then further
dialed down to 2ma/cm^2 for alpha and kept at 5 for beta. these lower current densities prevent that toxic spray from being too much of an issue and
the new alpha plating bath does not produce bubbles at either electrode.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
I made another thick one

|
|
|
mekanochemical
Harmless

Posts: 22
Registered: 15-11-2019
Location: South of heaven
Member Is Offline
Mood: always under 7
|
|
A little question about the cell running
Here the table salt came with a small amount of potassium iodide (around 40mg per kg), is it harmful to reaction or to electrodes?
Hue Hue Hue
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by mekanochemical  | A little question about the cell running
Here the table salt came with a small amount of potassium iodide (around 40mg per kg), is it harmful to reaction or to electrodes?
|
Its better to use dishwasher salt. Its much cheaper than than table salt and has no added spurious ingredients.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
| Pages:
1
..
44
45
46
47 |