| Pages:
1
..
40
41
42
43
44 |
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
^Considering I didn't say anything about using "salt" with muriatic, I'm not sure what you think you're critiquing.
In any case, this is tedious. If you have excess liters of sulfuric acid burning a hole in your pocket, obviously your problems are much different
from mine. Hydrochloric acid is easily concentrated by distilling it over any number of dehydrating agents -- H3PO4, CaCl2, H2SO4, take your pick. I
wish you luck in your efforts.
[Edited on 19-5-2021 by clearly_not_atara]
|
|
|
Keras
National Hazard
   
Posts: 896
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Then it might be best to boil it under reflux (to get rid of water vapour), and led the HCl gas through a calcium chloride trap. This is slightly more
complex than the sulphuric acid/salt generator, but has the definite advantage of not using any sulphuric acid.
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
"Upon treating sodium acetate with chlorosulfonic acid in a little acetic
anhydride there is formed acetic anhydride". K.E. Jackson "Smoke-forming chemicals". The reference is PYTASZ AND RABEK:
Przemysl Chem. 14, 529-35 (1930).
(sorry if it was already mentioned - it is really hard to re-read the whole thread again)
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
......it's a long thread , read 20 pages and didn't find any suggestion of doing a transterification of acetic acid with 4- toluene sulfonic acid in
toluene using a dean stark trap to trap water. to acquire acetic anhydride ....I hava 4 toluene sulfonyl chloride and want to hydrolize it to attempt
the procedure....no, no references only a theory...but would appreciate a method to hydrolyse th 4-toluene sulfonyl chloride to 4-toluene sulfonic
acid.......solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by solo  | | ......it's a long thread , read 20 pages and didn't find any suggestion of doing a transterification of acetic acid with 4- toluene sulfonic acid in
toluene using a dean stark trap to trap water. to acquire acetic anhydride ....I hava 4 toluene sulfonyl chloride and want to hydrolize it to attempt
the procedure....no, no references only a theory...but would appreciate a method to hydrolyse th 4-toluene sulfonyl chloride to 4-toluene sulfonic
acid.......solo |
There is no need for hydrolyzing Tosyl chloride(4-Toluenesulfonyl chloride),Just mix with dry Sodium acetate(1:2) and distill it !
The reaction is exothermic when started( 80-100c)
(Redistillation of product maybe needed if you want odorless and colorless Ac2O)
[Edited on 7-3-2022 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Quote: Originally posted by Waffles SS  | Quote: Originally posted by solo  | | ......it's a long thread , read 20 pages and didn't find any suggestion of doing a transterification of acetic acid with 4- toluene sulfonic acid in
toluene using a dean stark trap to trap water. to acquire acetic anhydride ....I hava 4 toluene sulfonyl chloride and want to hydrolize it to attempt
the procedure....no, no references only a theory...but would appreciate a method to hydrolyse th 4-toluene sulfonyl chloride to 4-toluene sulfonic
acid.......solo |
There is no need for hydrolyzing Tosyl chloride(4-Toluenesulfonyl chloride),Just mix with dry Sodium acetate(1:2) and distill it !
The reaction is exothermic when started( 80-100c)
(Redistillation of product maybe needed if you want odorless and colorless Ac2O)
[Edited on 7-3-2022 by Waffles SS] |
....thank you, what solvent would you use since tosyl chloride is insoluble in most solvents save benzene , ether and alcohol ( which might work for
both tosyl chloride and sodium acetate.).....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Sounds like waffles is describing a dry distillation to me.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
" Sounds like waffles is describing a dry distillation to me. "....you are right , that seems to be the case...thanks for pointing it out....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by solo  |
....thank you, what solvent would you use since tosyl chloride is insoluble in most solvents save benzene , ether and alcohol ( which might work for
both tosyl chloride and sodium acetate.).....solo |
As swim said,I mean Dry distillation.(i did this method many times with Tosyl chloride and even Benzene sulfonyl chloride)
Try 2:1 and 3:1 ratio of sodium acetate : Aryl Sulfonyl chloride
[Edited on 11-3-2022 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
so much bickering about acetaldehyde but nothing in this 1000+post thread on the oxidation of acetaldehy into acetic anhydride?
as for the construction its rather easy of what ive been told
i know one person who produced liters of acetaldehyde which he then stored as some type of ammonia adduct in freezer
a regular glass jar with metal screw lid has a hole drilled in one side of the lid, air pumped through here
the middle a piece of copper, copper wire, spiral wound is left hanging in the "air" in the jar
ethanol is put in the jar
and an outlet composed of copper tube- my guess is that soldering wouldnt be required, silicone should do plenty well
it does get quite hot however
prior assembly the copper is heated up, the reaction is autocatalytic, it heats itself as the ethanol reacts with the CuO or Cu2O, then reduced back
to copper, then oxygen in air delivered repeats the cycle
i recall something about silver or platinum being able to do the same with less heating- more desirable for making formaldehyde as that reaction tends
to blow up more easily
the acetaldehyde is condensed in a container with icewater
since this whole thread mentioning acetaldehyde has only mentioned the construction of acetaldehyde generator, we never got to the actually
interesting part, how do we go on from that to acetic anhydride? sodium persulfate? perchloric acid?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Well, for one thing, why would we choose a precursor (for Ac2O) which is a gas?
Acetaldehyde is a precursor to lots of interesting stuff, like 2-picoline, acetonitrile, glyoxal and chloral, but under most achievable conditions it
will oxidize at the 2-position rather than the 1-position, which means that obtaining acetyl anything is going to be hard. Instead, acetaldehyde is
interesting because it can be used to make other things.
Radical halogenation of acetaldehyde acetals should give acetals of acetyl halides. If the diol used can undergo a pinacol rearrangement, treatment
with HBr@HOAc might give acetyl bromide and pinacolone; hopefully there are no further condensations. This seems unnecessarily laborious, though.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
.....cyanuric chloride will produce the acetic acid chloride , this with acetic acid will give you acetic anhydride.....Note:the article of cyanuric
acid has been posted in the articles of interest in the Reference section.........solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i think i figured out why acetaldehyde has even been mentioned in the first place
it has been confused from acetone-glacial acetic acid reaction where acetone is reacted at high temperature to form ketene- which is a gas, a highly
deadly gas, this gas however is bubbled into glacial acetic acid in a totally closed apparatus where it then causes it to turn into acetic anhydride
as i see it, it would be best to have 3 glacial acetic acid traps for the ketene gas as well as ventilation.. ketene may be reacted easily with
ammonia gas just as a 100% scrubbing mechanism maybe?
video from dougs lab he explains hydrogen cyanide is dangerous in 50ppm where ketene is 5ppm or even less
so it was never about acetaldehyde, it does use a "ketene lamp" kinda construction, which acetaldehyde also does use, but very different setup!
the ketene in glacial acetic acid is decently attractive, but if one can just wetten anhydrous sodium acetate with glacial acetic acid, and distill
that, that would be a lot safer- i didnt quite see anything on temperature of this distillation, i would expect it to be decently high? sodium acetate
starts to decompose just over 150*C- so drying out NaAc has to be at most 150*C
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Acetaldehyde with tert-butyl hypochlorite should create acetyl chloride:
"tert-Butyl hypochlorite is a versatile and inexpensive oxidizing agent, that allows the conversion of alcohols to ketones, aldehydes to acid
chlorides, sulfides to sulfoxides, and hydroxylamines to nitroso compounds"
-https://www.organic-chemistry.org/chemicals/oxidations/tert-butyl-hypochlorite.shtm#:~:text=tert-Butyl%20hypochlorite%20is%20a,and%20hydroxylamines%2
0to%20nitroso%20compounds.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Lots of interesting transformations are possible with tBuOCl. It turns amines to N-chloroimines, aldehydes with sodium azide to acyl azides, allylic
carbons to allyl halides, incautious chemists to smoldering piles of ash, etc.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Well, there is this really annoying chemist who works 3 hoods away from me at work 
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
In the "acetonitrile method", is it really necessary to gas HCl? Couldn't you get a similar effect by adding an AcOH solution of H2SO4 or TsOH to a
solution of NaCl in AcOH/MeCN? Sodium chloride appears to be somewhat soluble in AcOH, and hopefully MeCN will not crash it out:
https://pubs.acs.org/doi/pdf/10.1021/ja01276a009
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Maybe this be unclear for this reaction but i had success on synthesis Methyl Phenyl Butenone(Not for P2P) by AcOH solution of H2SO4 in aldol
condensation of BzH and MEK instead of gassing HCl.
I think your idea is worth trying just as much as my idea.
[Edited on 19-5-2022 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
https://patents.google.com/patent/US3513180
"A mixture of 60 g. of benzoic acid and 0.5 g. of trimellitic anhydride in a 250 ml. flask was heated to reflux for 6
hours. Water vapor (about 2 ml.) produced by the dehydration reaction was condensed in a Dean-Stark trap. The product consisted of a mixture of
benzoic acid and benzoic anhydride."
If Bz2O reacts with NaOAc without a proton donor, I think Ac2O is the most volatile possible product. Benzoic acid is about three times as strong as
acetic acid.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  | https://patents.google.com/patent/US3513180
"A mixture of 60 g. of benzoic acid and 0.5 g. of trimellitic anhydride in a 250 ml. flask was heated to reflux for 6
hours. Water vapor (about 2 ml.) produced by the dehydration reaction was condensed in a Dean-Stark trap. The product consisted of a mixture of
benzoic acid and benzoic anhydride."
If Bz2O reacts with NaOAc without a proton donor, I think Ac2O is the most volatile possible product. Benzoic acid is about three times as strong as
acetic acid. |
As patent mentioned there is no need for NaOAc and Ac2O produced directly from Catalyst and Acetic acid.(I hope this route works because i was
unsuccessful in Phthalic anhydride + acetic acid)
| Quote: |
It has now been found that this objective may be accomplished by carrying out the dehydration reaction in the presence of a small amount of a
1,2-dicarboxylic acid or the corresponding anhydride which acts to catalyze the dehydration of the monocarboxylic acids.
Monocarboxylic acids that may be dehydrated by the process of the invention include saturated and unsaturated aliphatic, alicyclic, aromatic and
heterocyclic acids having from about 2 to 18 carbons, as well as derivatives of these acids. Examples are acetic, propionic,
caprylic, lauric, stearic, crotonic, oleic, linoleic, cyclopropanecarboxylic, cyclohexanecarboxylic, cyclohexenecarboxylic, cyclodecanecarboxylic,
benzoic, toluic, phenylacetic, cinnamic, 1-naphthylacetic, nicotinic and benzofuroic acids.
|
[Edited on 5-6-2022 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You are correct, the patent method can work directly with AcOH. But that doesn't lower the reaction temperature of 230-260 C, meaning that you need a
pressurized setup. I thought it would be more practical to use benzoic acid at atmospheric pressure for most members.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  | | You are correct, the patent method can work directly with AcOH. But that doesn't lower the reaction temperature of 230-260 C, meaning that you need a
pressurized setup. I thought it would be more practical to use benzoic acid at atmospheric pressure for most members. |
Thats true.
Monocarboxylic acids with a boiling point of less than 230 degrees do not lead to the formation of anhydride at atmospheric pressure,because below
this temperature, mentioned dicarboxylic acid(Phthalic acid) will not lose water and make anhydride itself.
The mechanism of this reaction is interesting for me.
I think each mole of Dicarboxylic acid react with two mole of Monocarboxylic acid and make 1 mole Asymmetric Dicarboxylic anhydride and above 230c 5
members Dicarboxylic anhydride regenerate and lead to formation of carboxylic anhydride.
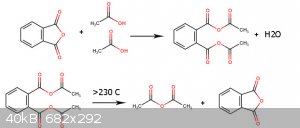
[Edited on 5-6-2022 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The preferred catalyst in the patent is trimellitic acid, which is not exactly something everyone has. But I'm pretty sure that it could easily be
produced by the action of permanganate on naproxen, which is 2-(6-methoxy-naphth-2-yl)-propanoic acid. Naproxen is also known as Aleve:
That permanganate reacts with naproxen is documented in this M.S. thesis:
https://smartech.gatech.edu/bitstream/handle/1853/33870/gibs...
but the products are not identified. However, it is known that phthalic acid is produced by the action of permanganate on naphthalene:
https://researchgate.net/profile/Bijudas-K/publication/31211...
so it is a natural extension to infer that trimellitic acid is produced by oxidation of the naproxen ring and alkyl chain. Permanganate is also known
to oxidize phenylacetic acid:
https://research.monash.edu/en/publications/heterogeneous-pe...
So this catalyst might be very easy to get after all. And the method should scale nicely.
|
|
|
BAV Chem
Harmless

Posts: 28
Registered: 9-5-2021
Location: In the middle of nowhere
Member Is Offline
Mood: Thoroughly confused
|
|
Quote: Originally posted by dicyanin  |
GB299342 looks interesting as sodium metaphosphates are commercially available OTC. Sodium hexametaphosphate is a common food additive (E452i) and
water softener, and sodium trimetaphosphate is used in construction. Calgon according to some sources is sodium polymetaphosphate
Na2[Na4PO3]6 but polycarboxylate to others. Still I've seen 1lb bags of pure sodium hexametaphosphate
offered for under 10 euros as a water softener.
The patent from 1927 claims "the production of acetic anhydride from a mixture of sodium metaphosphate, anhydrous sodium acetate, and infusorial earth
moistened with glacial acetic acid, the reaction being effected at 150-180°C". Sounds too good to be true, but it could work, and yield wouldn't
really be an issue considering the requirements. I've heard mixed results from a similar low-yielding process, heating sodium pyrosulfate with sodium
acetate.
|
That patent (see attachment) also claims that acetic anhydride can be produced by heating a mixture of glacial acetic acid and sodium
hexametaphosphate which doesn't work as far as I can tell.
Here's what I did:
127g of anhydrous sodium acetate and 170g of concentrated (~90%) phosphoric acid were thoroughly mixed and refluxed for some hours. I then distilled
off the concentrated acetic acid that was formed in the reaction and heated the remaining sodium dihydrogen phosphate to ~700°C in order to convert
it into some nice and glassy sodium hexametaphospate. This was broken up and ground to a more or less fine powder. Next all of the hexametaphosphate
and acetic acid was refluxed in a round bottom flask for another several hours. During this time the solid really caked to the bottom of the flask
(stirring would've helped). Finally I distilled everything to dryness but the vapour temperature never rose above 110°C. Everything I got was some
(nearly) glacial acetic acid with a freezing point of 10°C.
Glacial acetic acid should freeze at 16°C
and it's meant to boil at ~120°C.
Acetic anhydride boils at ~140°C.
That doesn't quite mean it can't work at higher temperatures but simply boiling sodium hexametaphosphate in acetic acid doesn't do it. I'm kinda
skeptical about the rest of that patent now.
Attachment: GB299342A_Original_document_20230331211754.pdf (1.5MB)
This file has been downloaded 267 times
[Edited on 9-4-2023 by BAV Chem]
|
|
|
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
I feel like this might be old news for some people here, but I just read this paper on the production of acetic anhydride from phosphorous pentoxide,
calcium chloride, and even simply sodium acetate. Of all the organic reagents I've seen, making acetic anhydride seemed difficult due to the
difficulty (for me) in obtaining some key reagents, but with this it seems like a no brainer.
https://www.ijraset.com/fileserve.php?FID=24897
Is this true? Is there a downside to this?
[Edited on 24-5-2023 by ManyInterests]
|
|
|
| Pages:
1
..
40
41
42
43
44 |