| Pages:
1
..
39
40
41
42
43
..
77 |
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
A few labsnaps ...
This first one is a solution of nickel chloride, a very lovely green color, but what I liked most was the layered, iridescent halo caused by the water
vapor coming off the warm, moist beaker. The camera doesn't do it justice!

These two are from some experiments I was doing with Liesegang rings. These test tubes contain gelatin impregnated with magnesium (left) and cobalt
(right), and a bit of ammonium hydroxide solution poured on top. A wave of cobalt hydroxide (?) appeared and traveled from the top of the tube to the
bottom, where it was reabsorbed. The magnesium hydroxide precipitated in a series of jagged, tangled layers.
 
This is some copper chloride which I left in a sealed container alongside a beaker of sodium hydroxide, to absorb acid fumes and water. Something odd
happened, though... the salts appeared to grow and effloresce, the feathery masses finally meeting up and merging. Cool!

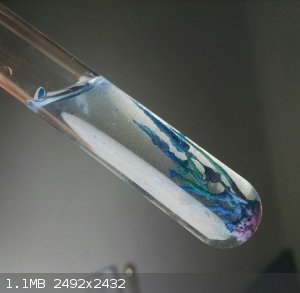
Finally, here's a picture from some recent experiments with "magic rocks" silicate gardens. I dropped a clump of moist cobalt chloride into some ~20%
sodium silicate solution, and over the course of about an hour this nice little tower sprouted!
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
the orange is acidified K2CrO7 the middle oxidized cyclohexanol and the left oxidized benzyl alcohol. the Chrome(VI) has me
nervous enough to actually wear gloves (knew people from Hinkley, CA).

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
  
About a year and a half ago I made some dimethyl oxalate, and haven't done anything with it since. I decided to check it out again, and remind myself
of the smell (sickly sweet brown butter solvent). I discovered that it had been slowly sublimating and crystallizing in the top of the jar. The photos
don't really do them justice, they look like shards of glass. The one on the jar lid and the larger ones on the paper are about 1 cm long.
|
|
|
stibium
Harmless

Posts: 7
Registered: 9-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  |
@Stibium,
Magnificent!
How did you do/make those? Via United State Patent n°US2544507A or else?
How is that phosphorescence activated, sunlight, UV light/laser and how long exposure?
How long does the phosphorescence lasts?
|
Hi,
I have always fascinated the chemistry of phosphorescence.
Here there is a digitized copy of a spanish old book specialized on phosphorescent alkaline earth sulfides:
https://archive.org/details/revistadelareal01madgoog
In the PDF file watch the pages 145 to 188, 406 to 465 and 578 to 640
I made my sulfides with help of this book.
Basically I made a suspension in water or ethanol intimately mixing 100 gr. of alkaline earth carbonate, 30 g. of sulfur, 1 gr. of sodium carbonate,
0.5 g. of sodium chloride and 0.02 gr. or less of bismuth subnitrate. I heated this mixture to evaporate the solvent, and put 10 gr of mixture inside
a covered crucible placed on a gas burner for 30 minutes.
The phosphorescence can be activated by sunlight or UV light. If the material is activated by exposure to sunlight five minutes, the phosphorescence
lasts half an hour.
To get better results the calcination temperature must be about 1000 or 1200 ºC
In these other links you can find more information about making Inorganic phosphorescent materials:
Alkaline earth sulfide phosphorescent pigments:
http://www.google.com/patents/US2544507
Phosphorescent Calcium Sulphide:
http://calcium.atomistry.com/calcium_monosulphide.html
Luminescence (easy synthesis):
http://www.teralab.co.uk/Experiments/Luminescence/Luminescen...
Many pictures and recipes:
https://www.flickr.com/photos/28617364@N04/9457791470/in/pho...
Phosphorescent materials (Spanish):
http://www.cientificosaficionados.com/tecnicas/fosforos.htm
Luminescence properties of CaS:Bi phosphors with special reference to fluxes:
http://shodhganga.inflibnet.ac.in/handle/10603/39477
|
|
|
Mabus
Wiki Master
  
Posts: 238
Registered: 3-11-2013
Member Is Offline
Mood: Energetic
|
|
Last year I added these cut steel pipes from some fridge scrubbers in some hydrochloric acid to get rid of the iron and get the copper. Little iron
dissolved and instead I got these small light blue crystals, about 1 mm in size. They don't seem to be soluble in water and they're pretty well stuck
on the iron pipe.

|
|
|
Starcruiser
Harmless

Posts: 18
Registered: 11-7-2015
Location: Orion Arm/Milky Way
Member Is Offline
Mood: No Mood
|
|
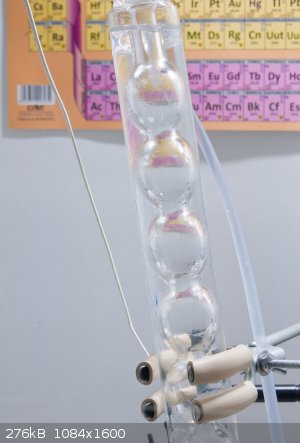
Methanol Distillation
I haven`t identified locally no other source of methanol other than the commonly available windshield washer fluid. It should be about 40-45% conc.
but I suspect that this is not really the case as long as the distillation started at a quite high temperature (~ 80 C).
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Really nice photo of the luminol precursor, zts16. Quite a set-up starcruiser!
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Rhodochrosite
Maybe for those of you who love minerals  For more photos from my collection,
visit my page CHEM.PIECEOFSCIENCE.COM For more photos from my collection,
visit my page CHEM.PIECEOFSCIENCE.COM
<img src="http://chem.pieceofscience.com/wp-content/uploads/2015/12/rodochrozit1.png" alt="rhodochrosite" height="600" width="900">
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
Nice crystal Hegi. Kind of Christmassy with the red and green.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
So I have no idea what this is probably some sort of Iron(III)Fluoride-Hydrate, due to its green color but could be anything else, too.
So I've been cleaning my crucibles that I brought home from the university over the holidays to clean them in the lab. That's how it started...
If you want to dissolve metals you boil them in Aqua Regia, if you want to get rid of Minerals, and that's what our expert for mineralogy at the
university told me once, you boil it in a mixture of H2SO4 and HF. So I took the crucible added some conc. HF to it and left it
for 2 days. It dissolved quite much of the compounds and probablya lot of my corundum crucible, too.
Then I changed it to the conc. HF + conc. Sulph Acid mixture and as that didn't help I took it a step further and even added some fluoride to it.
No it's hard to find anything on the solubility of HF in H2SO4 but it's something around 38% depending on the temperature. And
as it was getting warmer I had a constant stream of dry, gaseous HF coming of for 48h ! So I checked it every few hours and it was always fuming off
some HF. The mixture worked wonders and dissolved most of the stuff but I had placed everything in that stainless steel bowl and due to the cold
temperatures it's quite humid in my lab so the bowl slowly filled with humid HF vapours ...
I have to say I used that bowl for like 4 years now. Boiled acids, Lyes and lot's of burning stuff in there. It has been heated to hot temperatures so
often, left with conc. Acids for days and there was never ever any sign of rust, corrosion or any effect on it. It took the HF like 2 days to cause
what you see on the pictures. After one day those green crystals had formed and back then those were really beautiful crystals not like the amorphous
thing you see there.
I'm not even mad but just impressed how fast everything went. Not sure what it is but since there mostly fumes of HF present it should be some mixture
of Iron - Sulphate, Fluoride, Hydrate whatever. Really beautiful. I dried it and it yielded some brown and light green powders that are insoluble in
water. So looks like the Fluoride to me.

|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
That's vague;y remnicient of my basement sink after my usage of it for a few years...
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by stibium  | Quote: Originally posted by PHILOU Zrealone  |
@Stibium,
Magnificent!
How did you do/make those? Via United State Patent n°US2544507A or else?
How is that phosphorescence activated, sunlight, UV light/laser and how long exposure?
How long does the phosphorescence lasts?
|
Hi,
I have always fascinated the chemistry of phosphorescence.
Here there is a digitized copy of a spanish old book specialized on phosphorescent alkaline earth sulfides:
https://archive.org/details/revistadelareal01madgoog
In the PDF file watch the pages 145 to 188, 406 to 465 and 578 to 640
I made my sulfides with help of this book.
Basically I made a suspension in water or ethanol intimately mixing 100 gr. of alkaline earth carbonate, 30 g. of sulfur, 1 gr. of sodium carbonate,
0.5 g. of sodium chloride and 0.02 gr. or less of bismuth subnitrate. I heated this mixture to evaporate the solvent, and put 10 gr of mixture inside
a covered crucible placed on a gas burner for 30 minutes.
The phosphorescence can be activated by sunlight or UV light. If the material is activated by exposure to sunlight five minutes, the phosphorescence
lasts half an hour.
To get better results the calcination temperature must be about 1000 or 1200 ºC
In these other links you can find more information about making Inorganic phosphorescent materials:
Alkaline earth sulfide phosphorescent pigments:
http://www.google.com/patents/US2544507
Phosphorescent Calcium Sulphide:
http://calcium.atomistry.com/calcium_monosulphide.html
Luminescence (easy synthesis):
http://www.teralab.co.uk/Experiments/Luminescence/Luminescen...
Many pictures and recipes:
https://www.flickr.com/photos/28617364@N04/9457791470/in/pho...
Phosphorescent materials (Spanish):
http://www.cientificosaficionados.com/tecnicas/fosforos.htm
Luminescence properties of CaS:Bi phosphors with special reference to fluxes:
http://shodhganga.inflibnet.ac.in/handle/10603/39477
|
Thank you for those valuable infos...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Texium
|
Thread Split
2-1-2016 at 12:36 |
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|

upload a picture

image hosting over 10mb
My FeSO4.7H2O crystal druze . its about 1,2 inch across. its about 1,2 inch across.
image quality isnt wery good ill try to make better photos next time. ill try to make better photos next time.
Btw my 1st post 
[Edited on 3-1-2016 by crystal grower]
[Edited on 4-1-2016 by crystal grower]
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
These are my first bismuth crystals:

host images

picture hosting

gif image hosting
bismuth is my no.1 metal 
[Edited on 3-1-2016 by crystal grower]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Very nice indeed.
Care to post your process ?
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Very impressive Bi crystals!
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Thanks for compliment
The crystal growing process:
1. I created my Iron(II) sulfate by mixing 99.99% pure electrolytic iron with diluted sulfuric acid.(I didnt buy green vitriol bcouse I prefer
making the compounds myself ). ).
2.There is a problem with making iron sulfate crystals becouse some brown silt creates in the solution after some period of time . it is probably
some iron oxide so I come up with idea that you only need to add some extra sulfuric acid (it should react with the silt and create the FeSO4 again) -
it looks it worked properly in my case .
3.I grew some seed crystals by cooling the heated saturated solution and hung the best of them onto nylon.
4.then I drowned it into solution and after several days the crystals have created 
PS: sorry for my english feel free to correct me - I will learn something beside the chemistry at least    . .
[Edited on 4-1-2016 by crystal grower]
[Edited on 4-1-2016 by crystal grower]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Did an alkaline cleavage of piperine with KOH. My lil Kodak doiesn't have a macro mode darn it so the pics don't do justice to the BEAUTY of the
potassium piperate.

[Edited on 1-6-2016 by arkoma]

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
It looks like you have several grams there. Does it taste like pepper? 
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
haven't tasted it, the piperine base sure did.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice picture arkoma. Perhaps you should be wary, though, of posting pictures containing your address, unless you don't mind.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
sulaiman was kind enough to U2U me about it, BUT, I really got squat to hide and it would take 3 whole MINUTES to edit the photo. Fort Meade ALREADY
knows where we all live anyways. (Ft Meade=NSA HDQTRS)
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by arkoma  | | sulaiman was kind enough to U2U me about it, BUT, I really got squat to hide and it would take 3 whole MINUTES to edit the photo. Fort Meade ALREADY
knows where we all live anyways. (Ft Meade=NSA HDQTRS) |
Oh, yeah, they definitely do... 
I already put lab pics in the lab folder, but I really think my lab is pretty 
Anyways, Some chemical testing with ferro/ferricyanide and zinc cyanide.
Also the blue product is degraded Tetraamminecopper(II) acetate.
  
[Edited on 1-7-2016 by The Volatile Chemist]
|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
Fresh batch of Ammonium Nitrate


|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
@Nedshead, nice looking AN.
Just curious, what method did you use to make it?
[Edited on 9-1-2016 by greenlight]
Be good, otherwise be good at it 
|
|
|
| Pages:
1
..
39
40
41
42
43
..
77 |