| Pages:
1
2
3
4
5
6
7 |
CommonScientist
Harmless

Posts: 24
Registered: 7-2-2004
Location: In front of a faulty nitric acid still
Member Is Offline
Mood: Tense
|
|
I followed Haggis' instructions, but used my beakers, and only 350ml of solution, and added 10ml of reactant.
My results were somewhat better than his:

\"rules are for the guidence of the wise, and the blind obedience of fools\"
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
Good to know you can use less bleach, I did 2 2500 mL of 6% bleach runs, using 50-55 mL Acetone, and after multiple, very innefficient washes was left
with 90 mL, next time I will do 63 mL Acetone.
[Edited on 27-10-2004 by Mendeleev]
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
explicit_expletive
Harmless

Posts: 1
Registered: 17-11-2004
Location: Epsilon Persei III
Member Is Offline
Mood: No Mood
|
|
Low-tek
My experience has shown that great care must be taken to control the reaction so as to prevent decomposition of the reagents or catastrophic failure
of equipment. My advice is, if you so wish, to pre-cool the acetone and hypochlorite solution in seperate, closed vessels capable of withstanding
negative pressure such as a mason jar. I accomplished this by leaving a few jars in a standard freezer for thirty minutes or so.
Furthermore, I've also found it important to add/produce only small quantities of chloroform at a time using my... "equipment."
Glass is a good insulator, and freezers only get so cold. Thus, a limited amount of heat can be transferred to the surrounding air per given amount
of time. If this amount is exceeded because too large a quantity of material is reacted, then your target temperature will be forfeit.
~explicit_expletive
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
I don't think this reaction reqiures that much temperature control, very little in fact. The only thing I did with a 2.5L batch was leave the
flask with bleach in a bucket of ice 30 minutes before adding the acetone in two portions, leaving it to react on ice. The great thing about this
reaction is that it CAN be scaled up. Buy a trash can, 10 gallons of bleach, a liter of acetone, and about 50 lbs. of crushed ice, and you can
produce yourself about a liter of chloroform after drying, etc.
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
I have found that it's possible to do this at higher concentrations (using dry Ca(OCl)2 as hypochlorite source) if you are patient. The dry
powder and about 5 times its volume of cold water are mixed together, the reaction vessel immersed in cold water, and acetone slowly
dripped in. I improvised the dripping by poking a pinhole in the bottom of a soup can, setting it in a funnel, and setting the funnel in the neck of
the vessel. I set a timer so that I went to swirl the contents every 15 minutes, and added the acetone over a period of about 2 hours. I used somewhat
of an excess of acetone and let it sit overnight since a considerable amount simply evaporated while waiting to drip and I wanted to make sure all
hypochlorite was consumed for the next step.
The next step was getting something reasonably clear that can be filtered or separated, since there's a bunch of pasty calcium compounds left
after all the hypochlorite has been consumed by reaction with acetone. I added portions of hydrochloric acid and swirled until most of the solids had
gone into solution. There was no chlorine released. There's a healthy blob of chloroform at the bottom of the vessel waiting to be separated,
washed, and dried. It looks considerably larger than from previous attempts where I tried to work faster. I will probably repeat this process several
times over the next few days since I need to use up my calcium hypochlorite before its packaging completely deteriorates, though I don't have
much use for additional chloroform.
[Edited on 12-12-2004 by Polverone]
PGP Key and corresponding e-mail address
|
|
|
HNO3
Hazard to Others
  
Posts: 211
Registered: 10-11-2004
Location: America
Member Is Offline
Mood: No Mood
|
|
I did the readtion between bleach and acetone. I used an excess of bleach (I think). I got a yellow solution above a heavy (s.g.>1), milky (like a
colloid?), nonpolar (it splits like mercury when I hold a stirring rod to the side of the graduated cylinder it is in) liquid. It has no knock out
effect, but I do have a headache. When I heat it, it turns clear but yellow colored. It starts bubbling about 50*C, but I never had it at a full boil.
Could I have made CCl4? Or is it a mixture? What went wrong? (famous last words)
\"In the beginning, God...\" Wait a minute, God doesn\'t exist!!!!!!!!!! \"OK, in the beginning, ummm, hydrogen...\" Wait a minute, what about the
laws of thermodynamics? \"OK, in the beginning, ummm.....UMMMMM, what\'s left to choose from?
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
| Quote: | | It starts bubbling about 50*C |
Somewhat lower then chloroform, and significantly lower then carbon tet, most likely just acetone boiling off. Carbon tet is usually an impurity,
almost never a major constituent of chloroform made this way.
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
A little off topic but interesting:
| Quote: | Chlorine will readily react with trace quantities of bromide to form hypobromous acid.
HOCl + Br- ---------> HOBr + Cl- (4.21)
The speed of this reaction is such that it rapidly proceeds until there is very little bromide left. The hypobromous acid formed can then react with
organic matter reforming bromide which completes the cycle.
d[HOBr]/dt = (3.1x103 l/M-sec)[HOCl][Br-] (4.22)
Therefore, in the presence of bromide, the active halogen shifts from chlorine to bromine. This is significant, because bromine may react differently
with organic matter than chlorine does. For example, bromine is far more reactive with acetone, forming an order of magnitude more trihalomethane
(i.e., bromoform) than with chlorine. On the other hand chlorine gives higher concentrations of trihalomethanes with resorcinol than bromine. With
natural waters and aquatic fulvic acids, the presence of bromide usually supports an increase in THM production. Studies with extracted aquatic humic
material have shown a a sharp loss of TCAA and DCAA with increasing bromide concentrations (Croué, 1987). It is likely that some bromoacetic acids
and brominated THMs are formed in their place. |
From here. So I thought, "Hummm... that makes sense..." I had known that the series of reactivity was reversed with oxoacids, however I
thought, for instance, that if you wanted hypobromite, you would add elemental bromine to hypochlorite, and you would release chlorine. So I took 500
ml 10% NaOCl and added an excess NaBr, swirled and let sit for 25 minutes, the solution when I cam back was orange/red bromine may have been released
but the solution was definitely basic so it was definitely as hypobromite. I added 12 ml acetone and let react for 25 minutes, the reaction was much
faster then with the pure hypochlorite even though the reagents were near 0C. Upon completion of reaction the mixture looked the same as mixtures of
chloroform and hypochlorite that I get left over from those reactions. I had a graduated cylinder with 20 ml of chloroform from a previous run an
hour earlier with a layer of the remaining reactant mixture over it, I poured in the bromoform that I had made and it sunk straight to the bottom, it
even made its own layer separate from the chloroform layer, I didn't try forcing them to mix, I just figured the properties of bromoform and
chloroform were close enough that using a mixture of the two would pose no problems.
|
|
|
Protium
Harmless

Posts: 27
Registered: 25-12-2004
Location: Point Of Return (Subject To 15% Restocking Fee)
Member Is Offline
Mood: unenlightened
|
|
I used a five gallon bucket and dumped in a gallon of 5% store-bought bleach (NaOCl). I left bleach container in a cold (about 5 degrees C) room
overnight and then I added 216.1g of Ca(OCl)2. At such a low temp, a good portion of the Ca(OCl)2 did not dissolve, even with stirring.
I then added dropwise with stirring about 75mL acetone. About 15 minutes after the addition was completed, I checked the bottom layer for chloroform.
There was none.
I decided to wait a while. After about another half-hour, as I was walking by I heard a bubbling reaction. To my surprise the reaction was well
underway bubbling at 45 degrees C. At this temp; most of the Ca(OCl)2 was dissolved. I had already added the full theoretical amount of acetone, so it
was effectively like dumping in all of the acetone at once and the reaction proceeded too fast. In the end, there was no product at the bottom of the
bucket.
Conclusion: At a temperature of about 5 degrees C (or lower) , the reaction does not go forward at any appreciable rate. This is not to say that you
should not use cooling, but that there should be a balance between the exothermic heating of reaction and manual cooling of reaction.
It just depends on how you look at it...
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
Stirring helps a lot. My reaction was at around 0 C and when I swirled the flask for a little while it shot up to 30.
[Edited on 29-12-2004 by Mendeleev]
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
From 'Thorpe's Dictionary of Applied Chemistry' | Quote: | Manufacture of Chloroform from Acetone and Bleaching-powder.
-This is the process most generally employed. The method differs in minor detail with the various manufactures, but the following may be taken as
representatives. The reaction is carried out in a cast-iron still of about 800 gallons capacity, which is provided with stirring gear, steam-coils,
and cooling-coils, and is connected with a condenser; 300 gallons of water are run into the still, and 800 lbs of bleaching powder are added through a
manhole, which is then securely bolted down. During addition of the bleaching powder the mixture is very thoroughly stirred. (In some processes the
mixing is carried out in a separate vessel, and the suspension is strained from the larger unbroken lumps of bleaching powder before being allowed to
run into the still.) The container (A in the diagram shown on p. 78) is charged with 70 lb of acetone, which is then slowly run into the
bottom of the still by means of a valve B. The introduction of the acetone is accompanied by a rise in the temperature which is not allowed
to exceed 110 F., cooling being effected if necessary by stopping the flow of acetone and circulating cold water though the cooling coil in the still.
When all the acetone has been introduced the contents of the still are raised to 134 F. At this temperature chloroform begins to distill over. The
temperature is then very gradually raised to 150 F., so as to keep the chloroform readily distilling. Towards the end of the reaction the mixture is
stirred and the temperature raised until no more chloroform distills over.
The crude chloroform obtained is separated and purified first by agitation with concentrated sulfuric acid. This operation is carried out in the
vessel shown in the diagram ; 1,500 lb. of crude chloroform are introduced into the vessel and thoroughly stirred, by means of the agitation gear
shown, with 600 lb. of sulfuric acid. The stirring is continued until a sample of the chloroform when thoroughly shaken with pure concentrated
sulfuric acid does not impart the slightest color on the latter. The time required for complete purification is usually about 3 hours. The
chloroform is next separated from the sulfuric acid and finally distilled over lime. The yield obtained from the above quantities averaged from over
2,000 batches was 124 lb., the highest yield in any one case being 131 lb. Variation in yield is attributed to the varying composition of bleaching
powder, though doubtless other factors influence the result. Bleaching powder containing less then 33% of available chlorine gives unsatisfactory
results, while samples containing more then 35% of chlorine are also unsatisfactory. The best results appear to be obtained with bleaching powder
containing 34% of available chlorine. |
I didn’t even know this process was used let alone the main process used in the manufacture of chloroform at one time. I’ve also done some more
work with sodium hypochlorite, a mixture of 510 ml 10% NaOCl with 11 ml acetone, providing the reagents are pre-cooled to -10C when allowed to react
at these temperatures for an hour give chloroform in an adjusted yield in the 90 percentile range.
[Edited on 12/11/2005 by BromicAcid]
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Well for ease of use for everyone this translates too:
2 Liter Jar/Flask
1.2L Water
363g 34% Bleaching Powder Ca(OCl)2
38ml acetone
In a 2 Liter jar, add 1137 ml of water, 363g of bleaching powder with 34% Cl content is added slowly, this is very thoroughly stirred and then the
about 38ml of acetone is added. The container is cooled, so the temperature does not rise above ~43 degrees Celcius. NOTE 1 After ALL
of the acetone has been added, the temperature is raised to 57C, and then to 66C, until no more chloroform is being distilled over. NOTE
2
Average yeild of PURE chloroform: 55ml
NOTE 1: Depending on your materials, this reaction may be performed in seperate steps, then the chloroform seperated via seperatory funnel or syringe.
The chloroform is purified via distillation or with a suitable dehyrating/dessicating agent. This route may not be the most yielding, but it is the
most cost effective.
NOTE 2: This route can be done in a standard distillation set with an addition funnel (not sure of proper name) The only difference is that for some
of the reaction, the "boiling" flask must be kept cool. I would think the processes to go like this:
(see attachment)
And yes, I know I am THE WORST PAINT user ever. But I like MSPaint  . .
[Edited on 25-4-2005 by PainKilla]
[Edited on 26-4-2005 by PainKilla]
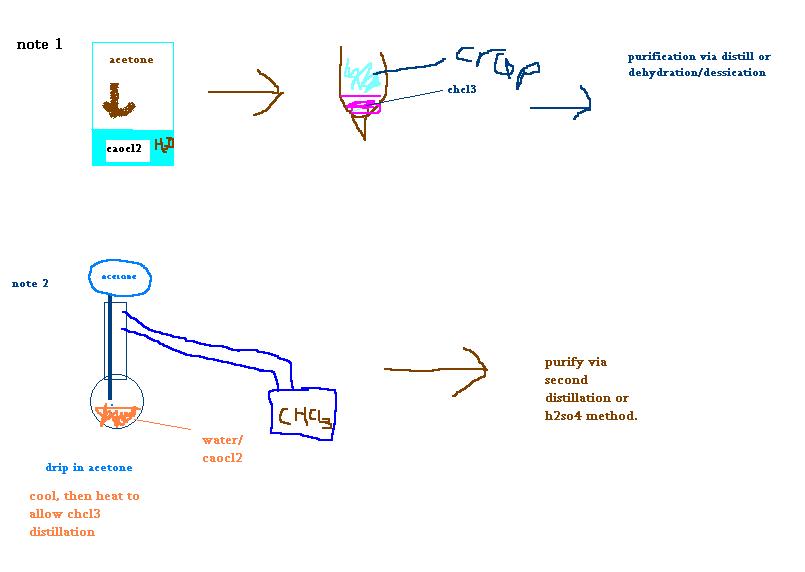
|
|
|
chloric1
International Hazard
    
Posts: 1142
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Crap
Painkilla, you regard the aqueous layer as crap. Not necessarily. If you where to use Methyl Ethyl Ketone instead of acetone you would get calcium
propionate along with your chloroform. Or sodium propionate if your use concentrated sodium hypochlorite with MEK. You concentrate the aqueous layer
and add 50% sulfuric acid to liberate propionic acid then extract in a suitable volatile nonpolar solvent. Ethanol would yield formates! Ethanol would yield formates!
Fellow molecular manipulator
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
I was just copying the procedure... It is rather cumbersome to do that, and well good point  . .
That is rather interesting info :p.
I am going to perform the whole thing (not your post) soon and give report on yields etc... as soon as my seperatory funnel comes in  . .
|
|
|
Hermes_Trismegistus
National Hazard
   
Posts: 602
Registered: 27-11-2003
Location: Greece, Ancient
Member Is Offline
Mood: conformation:ga
|
|
I've performed the oxidation of acetone by hypochlorites several times.
There is a valid reason that bleach is the hypochlorite of choice for this reaction for the amateur.
Bleaching powder is only partly(about 2/3'rds hypochlorite, the rest being insoluble (carbonates I think). After laboriously filtering out the
crap with a buchner funnel under vacuum, (and that takes a helluva long time due to the fineness of the particles after they break up from the
granules the come out of the canister in!!!!)
I added a six molar sol'n to the acetone and had massive precipitation resulting in nothing but a foam reminiscient of meringue...
The smell of chloroform is strong, but the chloroform itself is adsorbed onto the precipitate......calcium chloride I think...
also....whoever it was that thinks that they improved the yield of this reaction by adding more acetone is daft.
There is only one chlorine atom per NaOCl!!!
Matter is neither created or destroyed in chemical reactions....
Can't fight stoichiometry.
Oh look....I'm breaking my own rule about arguing with.....this guy
Arguing on the internet is like running in the special olympics; even if you win: you\'re still retarded.
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Personally the idea of using calcium hypochlorite sounds appealing, despite it not being the only thing in commerical bleaching powder it still
supplies more hypochlorite on a volume to volume ratio then commerical OTC bleach. However there is the problem of the insolubles, being that this
was once an industrial process it seems quite proven that a distillation straight from whatever you have after reaction does indeed work, but the
beauty of the reaction between acetone and bleach is the easy recovery of the product, although it should really be distilled anyway.
Polverone seemed to be on the right track when he reacted acetone with Ca(OCl)<sub>2</sub> dropwise to prevent excessive heating then
reacted the resulting product with HCl to dissolve the carbonates and other materials present facilitating the easy recovery of the liquid chloroform.
|
|
|
hdcwr0x2
Harmless

Posts: 8
Registered: 14-5-2005
Member Is Offline
Mood: No Mood
|
|
I don't mean to derail the thread, but I've got a couple of questions. I've made a small quantity of chloroform using acetone as the
the excess reagent (performed reaction in a large bucket without adequate measuring apparatus. I know, I need to invest in glassware but that's
beside the point. After about 30 minutes, the solution began to turn cloudy, and got warm as the ice I had added earler melted away. I cooled the
reaction vessle by submerging it into cold water. After a couple hours, the mixture had mostly seperated into 2 layers. I decanted most of the top
layer and poured the bottom one into a glass jar for later analysis. I left it in the freezer overnight, and when I looked at it this morning, there
was a layer of ice over a liquid layer. (There was still some water, because I didn't have the tools to extract the bottom layer completly.) I
saved the liquid and threw away the ice. Is what I have left fairly pure chloroform? I was worried about acetone contamination, but I had assumed
acetone to be less dense than water or chloroform, and I was right. Any acetone left over from the reaction would have been poured off when I decanted
earlier right?
Also, I am storing the chloroform in a completly dark freezer right now. Will this prevent decomposition into phosgene, or will I need to add
methanol?
[Edited on 10-10-2005 by hdcwr0x2]
|
|
|
nitroglycol
Hazard to Self
 
Posts: 56
Registered: 28-10-2005
Location: close to the centre of North America
Member Is Offline
Mood: curious
|
|
| Quote: | Originally posted by Haggis
With this experiment, I acutally found a use for my products, and a productive, legal one at that! A friend's little brother had to collect
bugs for his biology class. What else to use for the killing jar besides chloroform? It works very nicely, and is a lot quicker than the
'acetone free nailpolish remover' (ethyl acetate) that they told them to use. |
I've used chloroform in a killing jar once, and it seemed to work well; more often I've used Quick Start (which I believe is an ether-hexane
mixture), simply because it's more readily available than other agents (you can buy aerosol cans of it at Canadian Tire and similar places). But
maybe I'll try making chloroform between now and next summer and see how they compare.
[Edited on 5-11-2005 by nitroglycol]
|
|
|
Douchermann
Hazard to Others
  
Posts: 117
Registered: 11-10-2005
Location: Illinois, USA
Member Is Offline
Mood: No Mood
|
|
I'd like to point out that if you use an ice bath for the reaction, you have to bring it up to room temperature after all the acetone is added.
Keep it below 20C while you are adding the acetone (like 1ml, then stir, then 1ml then stir) and after its all added, stir it a little more then put
it on a table and slowly let it warm up to room temp. The reaction will procede as normal and may heat up to about 30-35C. You will be left with
nice clear TCM and quite a bit of it at that.
It\'s better to be pissed off than to be pissed on.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
HmMmMm?!?
Sorry if this was covered but does chlorine in water and acetone make CHCl3?
If it does TCCA could be added to water and acetone to make CHCl3, but not only that but also acetic acid (YAY), and C3H3N3O3
now the acetone and NaOCl forms; sodium hydroxide, sodium chloride, and sodium acetate, HOCl and acetone should or could form; di hydrogen monoxide,
hydrochloric acid, and acetic acid. as you can see HCl forms and that would make the reaction go faster untill a point at which no more HCl could
disolve, but this might be problematic since i am sure it would heat up, and it should be cooled, but i think i would still rather get some fairly
conc acetic acid, with not much of an effect on the yeilds for the TCM
So does anyone know if it does?
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
| Quote: | | Sorry if this was covered but does chlorine in water and acetone make CHCl3? |
Chlorine + Acetone + Water
gives chloroacetone, a potent tear gas type chemical. The haloform reaction requires basic conditions, these are provided by many salts of
hypochloric acid by their hydrolysis to give a hydroxide and the free acid.
|
|
|
ThermiteFiend
Harmless

Posts: 1
Registered: 15-7-2006
Member Is Offline
Mood: No Mood
|
|
   BE EXTREMELY CAREFUL!!! while chloroform *can* be used as a knock-out drug, the
dose required is pretty high. It is very easy to cross the line from knock-out to lethal. if you really want to pursue something of this nature, i
reccomend finding a compound that is a lot more managable, and not as easily lethal BE EXTREMELY CAREFUL!!! while chloroform *can* be used as a knock-out drug, the
dose required is pretty high. It is very easy to cross the line from knock-out to lethal. if you really want to pursue something of this nature, i
reccomend finding a compound that is a lot more managable, and not as easily lethal
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
After reading through this thread a few times, I've noticed one crucial step in this haloform reaction is being omitted--the addition of acid after
the initial addition of acetone to bleach.
Think of NaOCl as a source of Cl+ (or as NaOH and Cl2--where there is an occasional dipole in Cl2):
(CH3)C=O(CH3) --(NaOH)--> H2C=C-O(-)CH3 <--> H2C(-)C=O(CH3)
H2C(-)C=O(CH3) + Cl+ --> (CH2Cl)C=O(CH3)
This step repeats three times on one carbon, since with each chlorination it becomes even more reactive. The next step explains the "fogginess" :
:OH + (CCl3)C=O(CH3) --> CH3COOH + :CCl3 <--> CH3COO(-) + HCCl3
A proton exchange occurs, so some of the chloroform remains in solution until you add more protons so they don't have to be shared (i.e., pour in
some acid!). The carbonate is your fogginess.
I have done this reaction with Acetophenone, but not with Acetone. The yield was one mol Chloroform/mol Acetophenone + 1 mol Benzoic Acid. I
imagine yields of Chloroform would be even better with Acetone, since there are *two* methyl groups adjacent to a carbonyl (therefore, 6
chlorinations take place, and 2 mols Chloroform are formed from each mol of Acetone).
Even better, upon addition of acid, the Sodium Carbonate should form CO2, which conveniently bubbles away, leaving you with a sodium acid salt and
hopefully more chloroform.
Oh yeah, adding acid to bleach isn't always safe; chances are there will be excess bleach, so make sure to stay up wind and avoid chlorine fumes.
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | I imagine yields of Chloroform would be even better with Acetone, since there are *two* methyl groups adjacent to a carbonyl (therefore, 6
chlorinations take place, and 2 mols Chloroform are formed from each mol of Acetone).
|
No, as only the first methyl will react. There is a big difference between a carbonyl C=O of a aldehyde or ketone, and that of a carboxylic acid or
its salts.
Adding acid does nothing for yields. Outside of keeping the reaction mixture from getting too hot, the next best thing for increasing yields is to get
the chloroform dissolved in the aqueous layer. While the inorganic salts help reduce the solubility of chloroform, a fair amount does go with the
water.
Distillation can be used to get it out, but be aware that CHCl3 will react with base. From that standpoint neutralizing the solution will help yields.
Ehen I was doing it I had access to very low cost solid CO2, I would just toss bits of dry ice in until the pH and fizzness showed all the base was
now NaHCO3. Later I didn't bother with that, instead I made a flash distillation rig where a thin stream of the aqueous solution would vert quickly
be heated above the boiling point of chloroform. It was in effect a fractionating column jacked with boiling water, with the mix injected part way
down the column. It would be heated very rapidly to the point the CHCl3 came off, keeping the time it was exposed to hot alkali to a minimum.
The organic acid formed can be as useful as the CHCl3. Acetone gives acetate, as does isopropyl alcohol at the cost of an extra mole of hypochlorite.
Ethanol give formic acid salts, while MEK gives mostly propanate.
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
| Quote: | Originally posted by Polverone
p-DCB is supposed to react with aqueous ammonia in the presence of copper, but only at elevated temperature/pressure. I've tried quite a few things to
see if I can provoke it into reacting under more easily achieved conditions. Stirring/heating with alcoholic KOH? No. Stirring/heating with strong
NaOH solution? No. Stirring/heating with strong NaOH and PTC? No. Boiling it with alcoholic AgNO3? No. Boiling it with copper sulfate and household
aqueous ammonia? No. Dropping it onto molten KOH? No. Stirring it with methanol and aluminum amalgam? No.
On the bright side, it appears that p-DCB is a relatively high-BP solvent, inert to many reagents. Too bad it sublimes so easily.
Hmm. Refluxing with molten sulfur? I might have to give that a try... |
agreed, I`ve been trying a few of these this morning also, nothing of any significance occured either, although I read that hydrolysis NaOH/KOH will
produce the Ortho species rather than the Para.
Conc H2SO4 will be my next experiment (and anything else I can throw at it).
nevermind, that Nor a nitrating mix seems to do anything beyond distill itself up the test tube and smell of mothballs, maybe Mn2O7 might give a
result?
at least Moths won`t be a problem in Lab for a few day 
[Edited on 17-10-2006 by YT2095]
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
| Pages:
1
2
3
4
5
6
7 |