| Pages:
1
2
3
4 |
Mister E
Harmless

Posts: 18
Registered: 10-3-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  |
I find it difficult to believe that butane would leave more residue than limonene which has a much higher b.p.
I can imagine butane residues being more sensitive to detection than limonene,
due to the molecular similarity between limonene and THC,
and the b.p. difference between butane and the product..
|
Yes, by all accounts it's easier to remove butane than limonene. This is not about ease of extraction.
This is about residual taste. Butane can make some very sensitive. That's why the advent of butane less pipe lighters (hemp wick)
I wonder most about the true BP of THC and other cannabinoids. I guess I'll know better by next week.
Thanks for actually following my intent of this post, to talk about cutting edge extraction techniques rather than just arguing how to do the same
butane and CO2 extractions that were perfected close to a decade ago.
|
|
|
Mister E
Harmless

Posts: 18
Registered: 10-3-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sidmadra  | I find this a little funny and felt that it needs to be pointed out, since nobody has done so in the last 3 pages - people are arguing about CO2
extraction, and have been posting about it since the first page, when that isn't even what OP was asking about.
It was pretty clear to that he was asking about purifying cannabinoids (separating out the low-mid BP terpenes from the high BP cannabinoids),
POST-extraction, since he was talking about short path distillation. You know, taking the sticky wax and refining it into a nice white powdered
THC/CBD/Cannabinoid mixture. His entire first post is about distillation and boiling points, which being a chemistry topic works here. There isn't
any mention or question of the extraction process, yet people are here arguing over CO2 extraction.
|
Yup, thanks for trying to bring it back on topic. I hope once I'm running and start posting results maybe people get involved in the conversation I
wanted to have.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by Mister E  | Quote: Originally posted by Sulaiman  |
I find it difficult to believe that butane would leave more residue than limonene which has a much higher b.p.
I can imagine butane residues being more sensitive to detection than limonene,
due to the molecular similarity between limonene and THC,
and the b.p. difference between butane and the product..
|
Yes, by all accounts it's easier to remove butane than limonene. This is not about ease of extraction.
This is about residual taste. Butane can make some very sensitive. That's why the advent of butane less pipe lighters (hemp wick)
I wonder most about the true BP of THC and other cannabinoids. I guess I'll know better by next week.
Thanks for actually following my intent of this post, to talk about cutting edge extraction techniques rather than just arguing how to do the same
butane and CO2 extractions that were perfected close to a decade ago. |
OK, let's talk about the OP topic, limonene for use as an extraction agent of the target substances.
But let us discuss the whole enchilada, there is more in play here than TASTE. Hemp wicks? Forsooth!
Yes, as an entrepreneur pushing, er, I mean SELLING the extracted product/commercial extraction equipment, all that matters is market acceptance.
Taste would matter there.
I am more concerned with the systemic effects of the unintended consequences.
Every time I see something with an aromatic structure headed for the lungs, especially if it's going to be heated, oxidized, polymerized or otherwise
altered on the way there? I wonder what is really eding up in those nice pink alveoli.
So what are the pyrolysis products of this aromatic solvent? How are these chemicals biologically active? Anything teratogenic? Tiny particles going
all the way to the alveoli and STAYING THERE? Until and unless a phage cell gets them, and if so, how do phage cells react to this new dietary
supplement?
Here is the first 10 minutes of Google research- You've been breathing limonene pyrolysis products in the air near wood kilns, apparently.
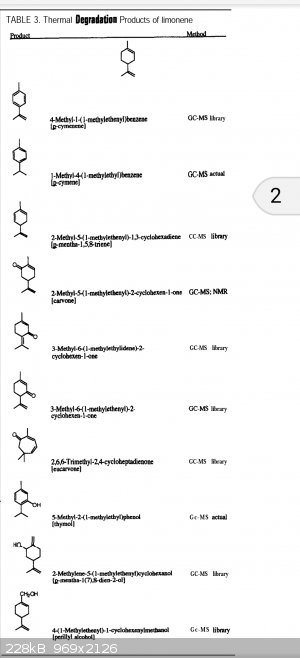
Attachment: ja_mcgraw001.pdf (212kB)
This file has been downloaded 484 times
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Maybe this thread can be split in a limonene and a CO2 thread?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Could be.
What does OP think about the idea?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
ISTR that limonene is a major product from the pyrolysis of scrap rubber tyres.
|
|
|
Sulaiman
International Hazard
    
Posts: 3782
Registered: 8-2-2015
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | | Quote: | If the sale and/or trade of the product is not allowed,
what is the benefit of regular extraction/concentration ?
(once or twice as an experiment I understand) |
I'm aiming for CBD, not THC. |
In that case, I volunteer my waste disposal services.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I don't think it is easily possible to separate the two, THC won't be a waste product.
The plant material started with doesn't contain THC
|
|
|
Mister E
Harmless

Posts: 18
Registered: 10-3-2018
Member Is Offline
Mood: No Mood
|
|
I'd be happy to see the CO2 conversation separated from the limonene conversation. I was thinking last night that once my last piece comes in next
week I was going to start a new thread with limonene in the title so as to try and keep it on topic a little better.
I have no issue with people talking about CO2 extraction and am curious how subcritical extraction would work for cannabinoids. Everything I've heard
is that subcritical works for terpene extraction, not canabinods. I do feel there is plenty of room for both conversations but my original topic has
gotten rather lost.
|
|
|
Mister E
Harmless

Posts: 18
Registered: 10-3-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bert  | Quote: Originally posted by Mister E  | Quote: Originally posted by Sulaiman  |
I find it difficult to believe that butane would leave more residue than limonene which has a much higher b.p.
I can imagine butane residues being more sensitive to detection than limonene,
due to the molecular similarity between limonene and THC,
and the b.p. difference between butane and the product..
|
Yes, by all accounts it's easier to remove butane than limonene. This is not about ease of extraction.
This is about residual taste. Butane can make some very sensitive. That's why the advent of butane less pipe lighters (hemp wick)
I wonder most about the true BP of THC and other cannabinoids. I guess I'll know better by next week.
Thanks for actually following my intent of this post, to talk about cutting edge extraction techniques rather than just arguing how to do the same
butane and CO2 extractions that were perfected close to a decade ago. |
OK, let's talk about the OP topic, limonene for use as an extraction agent of the target substances.
But let us discuss the whole enchilada, there is more in play here than TASTE. Hemp wicks? Forsooth!
Yes, as an entrepreneur pushing, er, I mean SELLING the extracted product/commercial extraction equipment, all that matters is market acceptance.
Taste would matter there.
I am more concerned with the systemic effects of the unintended consequences.
Every time I see something with an aromatic structure headed for the lungs, especially if it's going to be heated, oxidized, polymerized or otherwise
altered on the way there? I wonder what is really eding up in those nice pink alveoli.
So what are the pyrolysis products of this aromatic solvent? How are these chemicals biologically active? Anything teratogenic? Tiny particles going
all the way to the alveoli and STAYING THERE? Until and unless a phage cell gets them, and if so, how do phage cells react to this new dietary
supplement?
Here is the first 10 minutes of Google research- You've been breathing limonene pyrolysis products in the air near wood kilns, apparently.
|
An important thing to note is that limonene is one of the main terpenes already present in cannabis. Almost all hash oils contain a small percentage
of limonene already. Part of the whole point of using limonene as an extraction solvent is to keep it as close to the whole plant as possible.
|
|
|
Mister E
Harmless

Posts: 18
Registered: 10-3-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Tsjerk  | I don't think it is easily possible to separate the two, THC won't be a waste product.
The plant material started with doesn't contain THC |
It is possible to separate the two however in the united states pure CBD that is sources from marijuanna is federally illegal while pure CBD sourced
from hemp is legal.
Hemp of course will give you CBD but no THC. There is no need to seperate anything. Just extract all the cannabinoids and you will get a near pure
CBD oil with little to no THC included.
|
|
|
Mister E
Harmless

Posts: 18
Registered: 10-3-2018
Member Is Offline
Mood: No Mood
|
|
Perhaps but the limonene most people are familiar with and able to get is distilled from cold pressed orange peel oil.
|
|
|
zed
International Hazard
    
Posts: 2285
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Everybody and his uncle, is making "hash oil" hereabouts.
Legal, in accordance with local codes, but highly illegal in residential neighborhoods. Too many exploding houses.
Since there are no federal regulations being applied, in terms of purity, there is no standard.
I have a few acquaintances, in the trade, and I trust them..... not-at-all.
Butane, can blow you off the face of the planet... And, it may leave disgusting residues in the final product.
Isopropyl alcohol 99% ala Fred Meyer, is reputed to do a good job of extraction, but complete removal might not be easy. How much residue is OK?
Well, nobody knows.
Limonine makes no sense at all.
Since Oregon is an "Everclear" State... 95% Ethanol can be purchased OTC. Not cheap.
But, we know its qualities, and that a small amount of residue is probably harmless. Further, it can be recycled.
Technically, it should be possible to obtain "Tax Free" ethanol for hash-oil manufacturing purposes. But, that, isn't going to happen anytime soon.
The U.S. Federal government is tolerating, recreational pot manufacture and use in the Western States, because it would be hard to stop it. The
Federal Bureau of ATF, however, isn't going to start issuing permits, allowing Tax Free alcohol use, to manufacturers who are clearly violating
Multiple federal laws.
|
|
|
Sidmadra
Hazard to Others
  
Posts: 129
Registered: 17-2-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zed  |
Technically, it should be possible to obtain "Tax Free" ethanol for hash-oil manufacturing purposes. But, that, isn't going to happen anytime soon.
The U.S. Federal government is tolerating, recreational pot manufacture and use in the Western States, because it would be hard to stop it. The
Federal Bureau of ATF, however, isn't going to start issuing permits, allowing Tax Free alcohol use, to manufacturers who are clearly violating
Multiple federal laws.
|
I don't think the alcohol being tax free matters much. The last city I lived in, the guy running the local chemical/labglass supply store said that a
huge portion of his business was for people doing cannabis extracts. He always had a couple of barrels of Anhydrous nondenatured Ethanol and said
people would routinely come and buy 25L at a time. I think he was selling it for $300/25L if I remember right. In any case the tax status doesn't
matter a whole lot because most of the people using ethanol commercially in this manner are almost certainly using Rotary Evaporators to recover it.
This is typically the standard.
As interesting CO2 extraction is, I think it's over hyped - only a small fraction of companies are using them, because the cost of the extraction
units can be prohibitively expensive, and they usually don't allow much material to be processed at a given time. The upside is the CO2 is easy to
handle, cheap, and easy to evaporate.
CO2 extraction is impractical from an industrial standpoint because of the physics limitations that make it increasingly difficult to make huge
vessels that can also withstand high internal pressures. Industrially, the standard for large batch herbal extractions is, steeping the herb in a huge
vat of solvent(water or ethanol), running it through a continuous centrifuge to separate out the solids, followed by running it through some sort of
continuous evaporator to recover the solvent.
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Who said pipebomb again?

|
|
|
zed
International Hazard
    
Posts: 2285
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Back in the day, my small California college would have loved your supplier.
We were completely OK, with paying full retail... plus tax, for our anhydrous ethanol.
But, no chemical supplier, in our Rolodex, would sell to us outright. No one was willing to collect the tax. Too much trouble.
So instead, we were required to purchase tax free ethanol. Perhaps a total of a gallon or two, per year.
This Tax-free ethanol came with a meddlesome ATF inspector, who cruised by unannounced, every few weeks, to monitor our usage.
Well, it seems we were always using "Too much", and he tormented us relentlessly, over nothing apparent. Bastards!
If you are a sizable company, with a decent regulator, tax free might be OK..
Otherwise, as always, I suggest you pay the tax, and be free of interfering demons.
|
|
|
PhenethylamineMachine
Hazard to Others
  
Posts: 110
Registered: 22-3-2018
Member Is Offline
Mood: No Mood
|
|
https://patents.google.com/patent/EP1385595B1/en
Interesting extraction method using heated nitrogen, carbon dioxide, helium or argon.
|
|
|
Tsjerk
International Hazard
    
Posts: 3036
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I produced liquid CO2 for some time! There was a lot of really foamy solid CO2 under the plant material I was extracting. I screwed up though and
couldn't maintain the liquid long enough to do some real extracting because I tried to do the whole thing without PTFE tape and two parts got so stuck
without the tape I could never separate them to put tape in between them.
I could form liquid even with the heavily leaking joint, it extracted a bit of oil, but not much. I will order the two frozen parts again, as i will
have to order new parts anyway because the setup in the photo is to small to extract more than three grams of material anyway.
My search for a new apartment went so well I found a really nice place before I could really accumulate enough money to pay for the deposit, so I'm a
bit low on liquidity atm, so it is probably going to take some time before further experiments will be done. But hey, enough time to grow some plants.
|
|
|
Mister E
Harmless

Posts: 18
Registered: 10-3-2018
Member Is Offline
Mood: No Mood
|
|
Vacuum purge going on now. I've got a thick dark viscous oil that still smells of Limonene.
Extracted quickly, no problem separating most of the Limonene back out using vacuum. I'm now certain the higher BP for THC, 514C, listed under the
Dronabinol tradename at pubchem is correct and the more commonly excepted BP of THC at 157C is wrong. Some sources claim it is 157C at 0.05 mmHg but
that still only works out to 428.6C.
I plan on letting it purge a little more before I use Ethanol to recrystalize the plant waxes and remove them. Reports of final product soon.
|
|
|
Sidmadra
Hazard to Others
  
Posts: 129
Registered: 17-2-2017
Member Is Offline
Mood: No Mood
|
|
Nomographs are only a rough approximation in most cases, as the coeffecients are different for each molecule. The greater pressure/temperature
difference you are attempting to approximate, the more inaccurate it can become.
May I ask why you care about the BP of THC at atmospheric pressure? You'd never be distilling it at this temperature anyways, instead always under a
vacuum.
[Edited on 19-4-2018 by Sidmadra]
|
|
|
Mister E
Harmless

Posts: 18
Registered: 10-3-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sidmadra  | Quote: Originally posted by Mister E  |
May I ask why you care about the BP of THC at atmospheric pressure? You'd never be distilling it at this temperature anyways, instead always under a
vacuum.
[Edited on 19-4-2018 by Sidmadra] |
I am under a vacuum to remove the Limonene.
My first batch is not as free of limonene as I would like. I believe I need to move to steam distillation. Adding a 2 neck and a sep funnel next.
In the mean time there has been marketed concentrates with added Limonene for some time now so I'm not overly worried about this batch having some
leftover but this is not my final process.
|
|
|
|
Sidmadra
Hazard to Others
  
Posts: 129
Registered: 17-2-2017
Member Is Offline
Mood: No Mood
|
|
So you're trying to remove the extra limonene? What's the issue? Are you heating with an bath at a set temperature, or a set vacuum, and some limonene
remains that doesn't come over?
Try raising the vacuum or temperature slightly, or a lot, enough so that your product stays behind. When vacuum distilling off solutions of solvents
and high boiling compounds, raoult's law becomes notable in that the tiny amounts of remaining solvent demonstrate a higher boiling point as part of a
concentrated solution with high BP compounds. When I use my rotovap, I occasionally have to raise the vacuum(or temperature) a bit to get the last
remaining bits of solvent.
Like if I'm removing Chloroform as a solvent from my solution, 99% of it will come over at around 340mmhg and bath temp of 60C, however to remove the
last tiny amounts I have to turn the vacuum down as low as it will go. This is indicated by the fact that the residue in the flask is still liquid
when it should be a solid if all the solvent is evaporated. About 5 minutes after I turn the vacuum way up, the solution crystallizes. As a warning,
solvents evaporated under this condition won't condense on the condenser because the vacuum is too great, so they'll go into the vacuums oil if you
don't have a cold trap. This shouldn't be much of a problem for something non corrosive like limonene, but it's worth keeping in mind.
[Edited on 20-4-2018 by Sidmadra]
|
|
|
| Pages:
1
2
3
4 |