| Pages:
1
2
3
4
5
6
..
13 |
verode
Harmless

Posts: 32
Registered: 22-3-2005
Member Is Offline
Mood: No Mood
|
|
the NaBr with ice + Cl2+ some HCl
You get Br2 Its should be acid becose it could happen Br->HBrO3
The Br2(l) is heavy than water
|
|
|
HNO3
Hazard to Others
  
Posts: 211
Registered: 10-11-2004
Location: America
Member Is Offline
Mood: No Mood
|
|
I'm working on a method that allows absolutely no formation of halohalides. I also don't want to use H2SO4 because the heat spike
volitalizes so much Bromine. So, the current results.
I was not in a mood to measure very carefully and I didn't have my scale there, so its pinch of this, dash of that chemistry. I used Sodium
Bromide, then added 6% bleach and swirled the mixture until it dissolved all the sodium bromide to form an orange solution. To this was added citric
acid. Fizzing started immediately, but died down quickly, and the solution turned a deeper orange. Remembering H2O2 as an oxidizing agent, some was
added. The solution turned white and what little bromine was on the bottom dissolved. More citric acid was added, but the orange color did not
reappear. So, to decompose the peroxide, potassium permanganate was added (~5g). The solution turned purple, then a pond scum color, as a dense oily
<b>ORGANIC</b> settled to the bottom. That is when I left. Did I manage to make bromoform? Or is it some other chlorinated/brominated
organic? I don't know. The work continues.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Anybody tried the reaction of TCCA with sodium bromide solution ?
Evidently what results is sodium cyanurate
in solution , and bromine on the bottom .
See the patent GB1401120 . The file is attached to one of the recent posts in the hydrazine thread .
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
I tried the reaction. According to the patent, the TCCA/NaCl reaction produces appreciable free halogen only when the solution is heated, strongly
illuminated, or placed under reduced pressure. There isn't a lot of free halogen produced otherwise. The same seems to be true with bromine: the
sodium bromide/TCCA mixture instantly turns orange on addition of water, and you can smell the bromine, but there is no liquid bromine formed, nor any
visible bromine fumes. The liquid isn't the dark color expected of saturated bromine water.
On acidification with H2SO4, the reaction began with more vigor. This is not especially surprising; the TCCA seems to act like more conventional
oxidizers in the production of bromine. The addition of conc. H2SO4 heated the mixture, and after a couple small pipettes of acid, the mixture was
merrily fizzing out vapors. I would be interested in seeing what the effects of heating alone without acid addition are, but that will have to wait
until the fumes have cleared out of my workspace.
PGP Key and corresponding e-mail address
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
You might try putting a layer of ether
or methylene chloride on top of the mixture and setting it in sunlight .
The solvent should move the equilibrium to the right the same as heating or vacuum , and so will the sunlight .
Of course shaking it up once in awhile or stirring it should help too and the color and the bromine should end up in the solvent .
|
|
|
trilobite
Hazard to Others
  
Posts: 152
Registered: 25-2-2004
Location: The Palaeozoic Ocean
Member Is Offline
Mood: lonely
|
|
Many ethers are oxidised to esters by bromine, the reaction product HBr being also able to cleave ether bonds, so methylene chloride is better.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
The problem here is separating the two afterwards. They're both nonpolar and have about the same boiling point. Separation would be very
difficult without a good fractionating setup.
|
|
|
azaleaemerson
Harmless

Posts: 27
Registered: 8-4-2005
Location: AR
Member Is Offline
Mood: No Mood
|
|
Just make sure you don't get bromine onto your hands. I once was helping to move a few items off a bench in a friends lab at work one day. I
grabbed the capped bottle of bromine to move it to the shelves. The lid wasn't on tight and it ran over my fingers. Lots of water washing
didn't help much. My fingers were actually the minor pain. The thinner skin of the webbing was eaten away. First orange, then white, now a
scarred pink. This is one of those memories I won't soon forget.
azalea
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Don’t worry, I’m sure that most of us here know what we’re doing. Still, bromine burns are definitely something to avoid.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Nice  Looking forward to reading more about it. Looking forward to reading more about it.
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
ADP
Hazard to Others
  
Posts: 120
Registered: 4-4-2005
Location: USA
Member Is Offline
Mood: Productive
|
|
In to route to hydrogen bromide gas the reaction occurs in the following way:
NaBr + H2SO4 --> NaHSO4 + HBr(g)
My question: When one does this reaction wouldn't the HBr react with the remaining H2SO4 and since HBr is such a strong reducer, wouldn't it
be oxidized by the H2SO4 and free the bromine?
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Yes, that is what happens to some extent. Small samples of bromine are commonly prepared like this.
|
|
|
ADP
Hazard to Others
  
Posts: 120
Registered: 4-4-2005
Location: USA
Member Is Offline
Mood: Productive
|
|
If that is the case, is there a more desirable way to produce HBr gas without the contamination of free bromine?
I guess one could always perform the reaction and then heat the reagents until the HBr comes out of the solution. Also perhaps the H2SO4 could be
dilluted as well the the NaBr be aqueous solution
|
|
|
wa gwan
Harmless

Posts: 37
Registered: 15-4-2005
Member Is Offline
Mood: No Mood
|
|
Non-oxidising acid
The use of the non-oxidising phosphoric acid and a potassium or sodium bromide salt should produce bromine free hydrogen bromide gas.
H3PO4 + KBr ---> HBr + KH2PO4
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Whaddabout plain HCl?
Tim
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
Probably quite a lot of HCl impurities mixed with the HBr.
|
|
|
ADP
Hazard to Others
  
Posts: 120
Registered: 4-4-2005
Location: USA
Member Is Offline
Mood: Productive
|
|
Wa gwan thanks for the info I should have thought of that.
|
|
|
mantis
Harmless

Posts: 38
Registered: 17-7-2005
Member Is Offline
Mood: No Mood
|
|
You can produce bromine with conc. sulphuric acid and potasium bromide.
It reacts in two steps:
H2SO4+2KBr-->2HBr+K2SO4
now the concentrated sulphuric acid will oxidise the HBr to water and bromine.
H2SO4+2HBr-->Br2+SO2+2H2O
All in all you can say:
2H2SO4+2KBr-->K2SO4+2H2O+SO2+Br2
You can save oxidation agents like KMnO4, MnO2, H2O2, etc. It´s a very cheap producing methode.
[Edited on 17-7-2005 by mantis]
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
| Quote: | Originally posted by rogue chemist
"Causes irritation to the gastrointestinal tract. Symptoms may include nausea, vomiting and diarrhea. May cause abdominal pain, reduced urinary
output, low blood pressure, methemoglobinemia, convulsions, liver and kidney damage, and coma. Cyanosis may occur as a later symptom. Death may occur
from renal failure, within 1 to 2 weeks. Estimated lethal dose is 4 grams."
Sounds like something really great to have in food products.  The MSDS must be
exagerating... The MSDS must be
exagerating...
Source:
http://www.jtbaker.com/msds/englishhtml/p5576.htm |
I bought a loaf of "White Enriched Bread" at the supermarket recently. I happened to notice on the label:
BROMATE FREE
UNBLEACHED FLOUR
NO ARTIFICIAL PRESERVATIVES
So apparently bromates are used in baking some types of white bread.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Well, bromate is a reasonable oxidizer, no? Probably serves the same purpose as OCl, bleaching (hence unbleached flour).
Tim
|
|
|
mantis
Harmless

Posts: 38
Registered: 17-7-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Well, bromate is a reasonable oxidizer, no? |
yes, but it need a acid medium.
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Safer Bromine Source
This isn't exactly OTC, but pyridinium hydrobromide perbromide makes a good brominating agent, and it is also much safer to use if you have limited
space/ don't have a fume hood. The perbromide forms a rapid equilibrium with pyridinium hydrobromide and bromine, so the bromine concentration is
fairly low. However, it is convenient, safe, and easy to work with. I'm not sure what it costs, but I imagine that it will be worth it. It might
not be the best for working on a large scale, but I know that it's perfect for my purposes. You can run the bromination in GAA and you'll be all set.
Djerassi, C.; Scholz, C.R. J. Am. Chem. Soc. 1948, 70, 417.
|
|
|
Pommie
Hazard to Self
 
Posts: 70
Registered: 6-2-2005
Location: Australia
Member Is Offline
Mood: No Mood
|
|
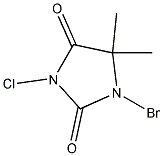
The only bromine source I can get hold of is 1-bromo-3-chloro-5,5-dimethylhydantoin
(BrClC<sub>5</sub>H<sub>6</sub>O<sub>2</sub>N<sub>2</sub> . Can this be used to produce bromine? . Can this be used to produce bromine?
Mike.
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
1-bromo-3-chloro-5,5-dimethylhydantoin
Sure, it looks like a strong brominating agent. The trouble is, that it will also be a chlorinating agent. Interesting chemical, actually, I would
very much like know if it would be useful. Some reactions don't require a specific halogen, just that the molecule be halogenated... so if a
few chlorines mucking about aren't a problem.... I imagine that you could put this to use, though I am hesitant to comment on the synthetic utility of
it.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
And if nothing else, the chlorine should displace the bromine and get you pure bromine, no?
Interesting molecule, looks like a variation on TCCA I'd say?
Tim
|
|
|
| Pages:
1
2
3
4
5
6
..
13 |