| Pages:
1
2
3
4
5
6 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
NC#6
Production of aluminium:
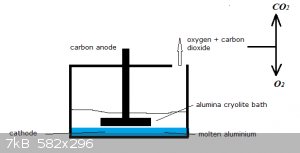
In one run 20.0 kg of Al is produced by electrolysis of an alumina/crylolite bath. The reaction is:
Al2O3(l) == > 2 Al(l) + 3/2 O2(g)
Due to the heat the carbon anode is partly oxidised by:
C(s) + O2(g) === > CO2(g)
After the run it's established the anode has lost 1.2 kg in mass.
The CO2 and O2 are separated.
Question:
How much oxygen (in kg) was obtained during the run and after separation from the CO2?
10 points.
[Edited on 1-9-2016 by blogfast25]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | The deadline for my question has passed, so I guess I ought to post the solution.
3.75 g of a metal chloride is dissolved in water and treated with excess aqueous silver nitrate, resulting in the formation of 10.66 g of precipitate.
Net ionic equation: Ag+(aq) + Cl-(aq) --> AgCl(s)
We obtained 10.66 g of silver chloride, which is 74.37 mmol. *If* the metal was monovalent, that would mean the molar mass of the metal chloride was
3.75 g / 0.07437 mol = 50.4 g/mol, which would give the metal a molar mass of 15.0 g/mol. This is not the molar mass of a metal.
If it's divalent, then the molar mass of the metal chloride would be 3.75 g / (0.07437 mol/ 2) = 100.8 g/mol. Subtracting the two chlorides gives
29.9 g/mol. This is between silicon and phosphorus, which are not metals.
Trivalent gives 44.9 g/mol, which is within experimental error for scandium (which is indeed trivalent).
Tetravalent gives 59.9 g/mol, which is close to cobalt or nickel, neither of which form tetrachlorides.
Pentavalent gives 74.9 g/mol, which would be arsenic, but arsenic is not a metal, and its pentachloride is not ionic.
Hexavalent would give 89.8 g/mol, which is close to yttrium and zirconium, neither of which have a +6 oxidation state. Similarly, rhodium and
palladium do not form heptachlorides, and tin doesn't form an octachloride. So the metal must be scandium.
[Edited on 1-9-2016 by DraconicAcid] |
Ouch! That was way above my current level, i didnt get close  . .
Onwards and upwards! More reading and studying needed then a crack at the other questions.
|
|
|
j_sum1
Administrator
       
Posts: 6325
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I like the look of these new problems bloggers. I am going to have a bit of fun doing them.
And, DraconicAcid, I thought the scandium chloride was genius. What a great way to pose a puzzle.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
All of this is an exceptional way to learn. I couldnt arrange enough time to do the last one, but having seen the answer i know i wouldnt have got it.
But learning is about being stretched, so i have learnt yet another way to approach a problem. While doing experiments is alot of fun, this kind of
thing is really useful in learning the basics, following a recipe in a book is mainly just practising technique.
|
|
|
Lefaucheux10
Harmless

Posts: 30
Registered: 28-8-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | NC#5
A sample is composed of potassium alum, sodium chloride and potassium nitrate. A 30.0 g sample is fully dissolved in water. To the solution 0.300 mole
of ammonia (NH3) is added and a precipitate is formed. The solution is then filtered and the filter cake washed. Filtrate and wash water
are quantitatively recovered and the combination diluted to 250.0 ml.
Several 25.0 ml aliquots of this stock solution are titrated with 0.9915 M hydrochloric acid in an acid/base titration. A significant average of 17.4
ml of titrant solution is used.
Questions:
1. Write the ionic reaction equation of the precipitation reaction, state symbols included.
2 points.
2. What is the weight percentage of potassium alum in the sample?
8 points.
[Edited on 2-9-2016 by blogfast25] |
Questions if you can respond without denaturating the problem :
1) is the alum anh ? or 12 H2O
2) is the NH3 added sufficiant to produce all the precipitate possible and not dissolve any of it ?
THX if informationS can be delivered
THX also if you can't ^^
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Lefaucheux10  |
Questions if you can respond without denaturating the problem :
1) is the alum anh ? or 12 H2O
2) is the NH3 added sufficiant to produce all the precipitate possible and not dissolve any of it ?
|
1) potassium alum is 12H2O.
2) NH3(aq) is too weak a base to dissolve the precipitate.
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
Very nice teasers, I'd appreciate you to continue posting them even after the end of competition. Some of them are very fun to solve 
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Neme  | Very nice teasers, I'd appreciate you to continue posting them even after the end of competition. Some of them are very fun to solve  |
I think alot of people would, they are also great for learning......BUT i suspect the problem is time, it must take a fair bit of time and effort to
come up with decent questions.
Its a bit much to expect people to put a huge amount of work in like this, on the other hand a few of the other Guru's might step in after the comp
and continue a non prize question thread.
What about the real noob questions like.......Why dont you use litmus paper to test the pH of conc sulphuric acid?
I cant be the only one who has tried it??
[Edited on 2-9-2016 by NEMO-Chemistry]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
What ?! You put litmus paper in conc sulph !?!?
Changes to Red(acid) then Black(burnt) then Little Bits(burnt to buggery) 
These questions are a bit hard for a complete noob - i'm certainly getting stretched a Lot trying to work them out.
Sadly us wormlings cannot see how simple and easy they probably are to those who fly high above us (in terms of chem knowledge).
Maths is very much involved in Chemistry - it's fundamentally necessary to work anything out.
Maybe make a Noob thread with noobs making the noob questions ?
Hardest thing will be where to pitch the level of the questions.
This must be a continual problem for teachers.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
What ?! You put litmus paper in conc sulph !?!?
Changes to Red(acid) then Black(burnt) then Little Bits(burnt to buggery) 
These questions are a bit hard for a complete noob - i'm certainly getting stretched a Lot trying to work them out.
Sadly us wormlings cannot see how simple and easy they probably are to those who fly high above us (in terms of chem knowledge).
Maths is very much involved in Chemistry - it's fundamentally necessary to work anything out.
Maybe make a Noob thread with noobs making the noob questions ?
Hardest thing will be where to pitch the level of the questions.
This must be a continual problem for teachers. |
Yes i think your right, really difficult to make a quiz for all people. But as long as a reasonable time is given then you have a chance to read up on
a concept and try and apply it.
Chemistry is a steep curve for sure, but each little win and snippet of hard won knowledge is well worth the effort needed.
In class i am up against mostly people who did 2 years of lower science, they have the advantage at the moment.
But i noticed a few just do the set work at school and then leave it at that. Few bother doing science at home, even a few who say they want to study
the Higher level and then uni.
So while my maths isnt as good as theirs, and my chemistry is currently below theirs. I still think i have an advantage, i try and research everything
to death.
I also try things out at home, mostly it goes wrong or is just basic things. But keeps me happy and sooner or later especially in practicals i will
have a better technique than the clever people. Well thats the plan anyway..
Glad your being stretched, shakes your confidence a bit when so many say they find the questions easy and are noobs. I find them far from easy but
nothing worth having is won easy.
Funny how it seems to be three main types, the ultra competitive who just want to win, then the want to wins for the prize and finally those who just
want to do it to learn regardless of any incentive.
Yeah i assumed every noob tried testing the pH of Sulphuric with litmus.
It dawns on you pretty quickly how stupid the idea is, and generally isnt something that should be admitted.
[Edited on 2-9-2016 by NEMO-Chemistry]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Been doing Chemistry as a hobby 2.5 years roughly, so still pretty much a noob.
These questions have taken me HOURS of thought, scribbling and research.
Exercises like these definitely stretch the understanding, and you come across information (during research) that you might not encounter any other
way.
What person, armed with conc sulphuric acid and litmus paper would NOT stick it in there to see what happens ?
answer: someone who isn't interested in finding stuff out.
For the Record, i do not want the prize - better a noober-noob gets it instead.
The fun is all in the learning and i got a lab already.
[Edited on 2-9-2016 by aga]
|
|
|
Texium
|
Thread Split
3-9-2016 at 15:43 |
Lefaucheux10
Harmless

Posts: 30
Registered: 28-8-2016
Member Is Offline
Mood: No Mood
|
|
I have finished all the problems wait for the results now ^^
thanks guys
|
|
|
MeshPL
Hazard to Others
  
Posts: 329
Registered: 20-4-2015
Location: Universe
Member Is Offline
Mood: No Mood
|
|
Will there be more questions posted? I must admit I'm kinda looking forward to them. The difficulty was ok so far, although the amount of quesrptions
could be higher...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I guess that anyone could post a question.
Maybe best to U2U it to bloggers for QA before posting.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MeshPL  | | Will there be more questions posted? I must admit I'm kinda looking forward to them. The difficulty was ok so far, although the amount of quesrptions
could be higher... |
Some people are never happy! Like i said before just be grateful people are taking ALOT of time and effort to do this, it just sounds a bit ungrateful
and a bit of a cheek to just EXPECT others to spend their time making you happy.
If your getting through them that quickly then you got to wonder if you fall under the noob category. I have no idea what level the original aim was
except 'noob'.
Aga i wouldnt call a noob as such, he is pretty clued up on a fair few things and found some of them a challenge. I am not finding them easy and i am
having to put alot of work and effort in.
Not trying to be funny with you but it worries me that people pestering will put an end to what is an excellent resource and way to learn.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Oh, definitely a Noob.
Yes, ok, done many things so far, yet Understanding WTF i did, why it worked/failed is quite another matter.
Asking for more Free stuff needs to be done nicely, seeing as it's for Free.
As i said, perhaps others might submit a question or two to the QA department.
I just did, albeit of lower quality/difficulty and of a sneaky nature.
[Edited on 6-9-2016 by aga]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Oh, definitely a Noob.
Yes, ok, done many things so far, yet Understanding WTF i did, why it worked/failed is quite another matter.
Asking for more Free stuff needs to be done nicely, seeing as it's for Free.
As i said, perhaps others might submit a question or two to the QA department.
I just did, albeit of lower quality/difficulty and of a sneaky nature.
[Edited on 6-9-2016 by aga] |
Blogfast is the sneaky question master lol. Yes i agree 100%, seeing as its free and has a prize attached then manners are required when asking!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MeshPL  | | Will there be more questions posted? I must admit I'm kinda looking forward to them. The difficulty was ok so far, although the amount of quesrptions
could be higher... |
At least one more coming up, maybe two. 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Reminder to Draconic Acid: I haven't received your marks yet! 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
NC#7
Analysis: method of Standard Addition
An analytical method yields a signal S (in mV) that is directly proportional to the concentration CR of a reagent R:
$$S=kC_R$$
A sample solution of R of unknown concentration is analysed and yields S = 60 mV.
To 250.0 ml of the solution, 0.05 moles of R and added and the new solution homogenised (assume no significant volume change happens). Analysis of
this solution give a signal strength of 120 mV.
Question:
What was the concentration of the original sample solution?
10 points.
[Edited on 11-9-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
NC#8
Reaction rate:
750 ml of 0.500 M HCl(aq) is reacted with a large excess zinc metal acc.:
$$Zn(s) + 2 HCl(aq) \to Zn^{2+}(aq) + 2 Cl^-(aq) + H_2(g)$$
The reaction is carried out with full stirring and at constant temperature. At the start of the experiment the chemist measures the rate of hydrogen
evolution to be 20 mmole/s (20 x 10-3 mol/s).
After some time t the chemist determines that 0.192 moles of HCl have reacted away. No significant change in volume has occurred.
Question:
What is the expected rate of hydrogen evolution at time t?
10 points.
Hint: the reaction rate v obeys the simple law:
$$v=k[HCl]$$
where k is a rate constant.
[Edited on 11-9-2016 by blogfast25]
|
|
|
ficolas
Hazard to Others
  
Posts: 146
Registered: 14-5-2016
Member Is Offline
Mood: No Mood
|
|
They are getting harder D:
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Great questions, and excellent excercise !
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
They sure are! Way past my ability  . Some bloody clever NOOBs about to answer
some of those . Some bloody clever NOOBs about to answer
some of those  . .
|
|
|
Neme
Hazard to Self
 
Posts: 86
Registered: 28-5-2016
Location: Czech republic
Member Is Offline
Mood: No Mood
|
|
Yep, if these are for noobs I don't dare thinking what I am 
I'll have to study something about this.
|
|
|
| Pages:
1
2
3
4
5
6 |