| Pages:
1
2
3
4 |
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
I assume most of the fellas wear X large, and everyone loves hot chocolate. X-mass list Ramium!
Good for you sir!
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Copper orthophosphate was made by reacting copper carbonate with orthophosphoric acid. The copper carbonate was from a pottery supply shop and the
orthophosphoric acid 85% lab reagent quality.
3CuCO3 + 2H3PO4 = Cu3(PO4)2 + 3H2O + 3CO2
- 10.8g phosphoric acid added to small beaker
- Measure out 15g copper carbonate and start adding in small amounts with hand stirring (acid should be in small excess).
- Bubbles (of CO2) and blue suspension forming. Lumps appear to form that had to broken up with the glass rod.
- Add 30g water to thin the solution and the remaining copper carbonate – the extra water seemed to speed up the reaction. Photo below.
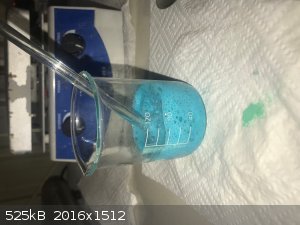
- Heat to near boiling for 10 minutes or so, then switch off heat but leave on hot plate to cool slowly. Left for the night.
- Next day – no more signs of any reaction. Blue ppt.
- Transfer to big beaker and wash with 3 portions of 300ml water by stirring, waiting for the ppt to settle, and decanting the wash water. pH between
3-4 after last wash. Photo after final wash below.

- Gravity filter and wash a few times in the funnel with water. Clear filtrate, Photo of wet ppt below.

- Dry on steam bath. First dry (3 hours) weight went from 29.3g to 15.7g.
- 2nd dry, 2 hours: Weight still the same, 15.7g.
- 2g used for test, 13.7g bottled. Photos of final product below, drying did lighten the color slightly.
 
Test product with hydrochloric acid – no bubbling, thus there do not seem to be a significant amount of carbonate left, and dissolves slowly to form
a light green solution.
|
|
|
| Pages:
1
2
3
4 |
|