| Pages:
1
2
3
4
5
6
..
22 |
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by Little_Ghost_again  | | As a side note what would the best way to analyze chicken shit with a GC be? I could then quantize the amount per g of each component, then compare
with some thats started to compost. |
GC as in Gas Chromatography? Ok, calm down already! Easiest way to determine ammoniacal nitrogen (i.e. ammonia + ammonium salts) would be dry
distillation of a know amount of chicken shit with an excess of solid NaOH. That will drive off all ammoniacal nitrogen as ammonia, quantitatively.
Capture the NH3 in water (quantitatively), dilute appropriately and determine the amount with acid/base titrometry (HCl titrant + methyl orange
indicator).
Constructing a neat little apparatus for the quantitative dry distillation of the NH3 is a doddle.
[Edited on 4-12-2014 by blogfast25] |
Ok I will give this a go.
I envy the bat cave person though, our bats are out of reach but according to the book contain 19% nitrogen in there poo  higher than chicken shit! higher than chicken shit!
Dont ask me, I only know enough to be dangerous
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by Little_Ghost_again  | | As a side note what would the best way to analyze chicken shit with a GC be? I could then quantize the amount per g of each component, then compare
with some thats started to compost. |
GC as in Gas Chromatography? Ok, calm down already! Easiest way to determine ammoniacal nitrogen (i.e. ammonia + ammonium salts) would be dry
distillation of a know amount of chicken shit with an excess of solid NaOH. That will drive off all ammoniacal nitrogen as ammonia, quantitatively.
Capture the NH3 in water (quantitatively), dilute appropriately and determine the amount with acid/base titrometry (HCl titrant + methyl orange
indicator).
Constructing a neat little apparatus for the quantitative dry distillation of the NH3 is a doddle.
[Edited on 4-12-2014 by blogfast25] |
Why would you need to dry-distill off the ammonia? The reaction proceeds much more readily with some water added, and couldn't you simply examine your
starting and ending volumes of water in the container used to capture the ammonia in order to find out how much water came over?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by No Tears Only Dreams Now  | | Why would you need to dry-distill off the ammonia? The reaction proceeds much more readily with some water added, and couldn't you simply examine your
starting and ending volumes of water in the container used to capture the ammonia in order to find out how much water came over?
|
Yes, you can add some water but it doesn't make much difference in terms of speed. And very high flow rates of NH3 can make capturing it less
efficient. Since as ammonia is still somewhat soluble in highly alkaline solutions you'll also lose a bit.
But why (and how) would you want to know how much water came over? Since as the ammonia too takes up volume (and mass) that's not so easy to do. It's
also unnecessary if you determine ammonia by titration.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Would it help if i Spell It Out ?
Anyone making HNO3 from a Chemical Nitrate that they bought, and some Sulphuric Acid that they bought, then distilled, will not be in any way
impressive, new, or worth any money.
Yes, making a nitrate salt from commonly available materials, and also making sulphuric, then distilling would be in with a chance.
Likely that a more Innovative synthesis would win though.
Distilling a Nitrate salt with conc H2SO4 is NOT the only way HNO3 can be obtained !
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
heating lead nitrate will give NO2,but I don't think lead nitrate is OTC
what about doing Ostwald process using a catalytic converter?
http://en.wikipedia.org/wiki/Ostwald_process
http://en.wikipedia.org/wiki/Catalytic_converter#Constructio...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Jan 1 has passed, so Submissions please, if anyone managed to make Nitric in a weird and wonderful way.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
You're not likely to get one, either. The requirements are just too strenuous. After all, any and all plausible and cheap alternative methods of
making nitric acid would have been researched to death during the world wars. Nothing significant emerged to the best of my knowledge.
[Edited on 4-1-2015 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
I have a plan! That might work. Maybe.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
And, of course someone may prove me wrong.....please, elaborate...?
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
I'm going to try it and post results. Soon. I hope. Just waiting for my neighbourhood to stop being on fire (wasn't me! Adelaide Hills fire if
anyone's curious)
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I'm still working on my stuff. I figured after the Fall semester ended that I'd have more time to work on this project, but that has not been the
case, unfortunately. I start classes again on the 12th, so if I'm able to finish, it will probably be before then. If not, well, I plan to keep
plodding on as time permits.
Economy in industry and on the lab scale are two different things. The Birkeland-Eyde route is too expensive on a commercial scale. In the lab,
though, it should take around $1-2 of electricity to make a liter of nitric acid.
|
|
|
Pasrules
Hazard to Self
 
Posts: 78
Registered: 4-1-2015
Location: Yellow Cake Deposit
Member Is Offline
Mood: Lacking an S orbital
|
|
This challenge might return us to the plasmoid in the microwave.
There is a thread all about it from 2005 (I think) but for summary basically put a container of water in a conventional microwave with some aluminium
and graphite electrodes (pencil) stuck to the underside of the lid to produce NO... You all know the rest.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WGTR  |
The Birkeland-Eyde route is too expensive on a commercial scale. In the lab, though, it should take around $1-2 of electricity to make a liter of
nitric acid. |
only if you have the right equipment which would be 100$ 
see this video
https://www.youtube.com/watch?v=2RRqIv4SoLg
but I must say that I was amazed how easily one could make tons of nitric acid this way.I actually rejected this same idea before as I thought that
making an electric arc would be difficult but I am a believer now
the microwave made nitric acid is this thread
http://www.sciencemadness.org/talk/viewthread.php?tid=4092
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Quote: Originally posted by CuReUS  | if you have the right equipment which would be 100$ 
see this video
https://www.youtube.com/watch?v=2RRqIv4SoLg
but I must say that I was amazed how easily one could make tons of nitric acid this way.I actually rejected this same idea before as I thought that
making an electric arc would be difficult but I am a believer now
|
It's a very interesting design, but I think it can be simpler than that. The fancy glassware isn't optimal or necessary. Since the magnets are
positioned in a skewed axis, the arc is rotated slightly. This isn't an issue, except that I'm the kind of person who straightens crooked wall
hangings. The power supply sounds like a high frequency unit, but I can't tell since it is cleverly concealed throughout the video.
The original design was essentially an iron container full of firebrick:
  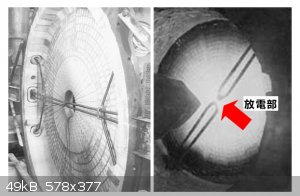
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Seems that people are actually very inventive at times.
I feel that there could be a few New Routes on their way.
We shall see.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Ah.
Slight Ethical dilemma.
In light of the recent EU-wide laws restricting HNO3 possession to 3% and the ongoing clampdown on chemistry in general, i feel that all and any
submissions should be posted in Whimsy, which would make the Resulting processes unavailable to Public view, yet leave them available to SM members.
If a Mod happens to be passing, please lock this thread.
A new thread is already open in Whimsy.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WGTR  |
The power supply sounds like a high frequency unit, but I can't tell since it is cleverly concealed throughout the video.
|
Is it a step up transformer or something like that
this youtube channel has many videos with different electrical devices so we can ask him about the power supply
https://www.youtube.com/watch?v=l4aI2Oiz1ps
even I had our european members in mind when I said that one could make tons of nitric acid this way.the UK laws are very strict nowadays 
[Edited on 5-1-2015 by CuReUS]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I suppose i really should ASK the mods and general population whether they think any new HNO3 synths should be made public or not.
It's not My website after all.
Comments/Opinions please.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Are you concerned with your own liability in offering prize money, or that others may get arrested trying to duplicate the experiment? Or are you
concerned about people who may misuse the information?
The contest could be reworded to only require 3% concentration, but in larger quantities. If one can make 3%, he or she can also make 30% anyway.
I'm here in the US, where it is still legal to own things like nitric acid, hydrogen peroxide, cars that get 10mpg, and Gatling guns.
At the same time, I'd like to think that I consider others before doing things. Certainly, if people think that posting this kind of information is
detrimental, then I will consider that.
|
|
|
Pasrules
Hazard to Self
 
Posts: 78
Registered: 4-1-2015
Location: Yellow Cake Deposit
Member Is Offline
Mood: Lacking an S orbital
|
|
I would love it to remain public as long as it is no risk to your liability, alot of people could really do with a procedure to make this stuff and so
far the only method i've seen come up is by using a microwave.
If criminals wanted to make this stuff they would take much easier routes with chemicals that include nitrates which we have excluded because we want
a challenge.
[Edited on 5-1-2015 by Pasrules]
Atropine, Bicarb, Calcium.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
My concern is primarily for the SM website.
I'd hate it to be taken down due to some random HNO3 process being posted at a time when the Powers that Be are taking steps to reduce public
possession of the same.
As i said, it's not my website, but then, if Polverone et al take issue with anything posted, i suppose they have the power to remove it.
Perhaps i'm being overprotective of my favourite website.
[Edited on 5-1-2015 by aga]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | I suppose i really should ASK the mods and general population whether they think any new HNO3 synths should be made public or not.
|
the idea is not actually new ,someone already thought about it before
http://www.sciencemadness.org/talk/viewthread.php?tid=32375#...
Quote: Originally posted by aga  | My concern is primarily for the SM website...
...Perhaps i'm being overprotective of my favourite website. |
really ?  i thought your favourite website was 9GAG i thought your favourite website was 9GAG 
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Aga, I don't know about privatizing information, however, your challenge is/was not really doable. I mean, you refused the use of high voltage, and
specialized equipment (quartz tube). These two things alone inhibit you from any process but pissing in a dirt pile until you get nitrate in the next
few years.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The appear to exist Other routes, not just the commonly used ones.
We'll have to wait and see if anyone successfully used one.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
The domain registration for this site, the hosting provider, and I myself are all located in the USA. The Powers that Be in the USA do not have power
of prior restraint over publication of information about firearms, propellants, high explosives, poisons, or other potentially dangerous items. They
can't even stop newspapers and blogs from publishing leaked top-secret government documents. I have no fear of a public HNO3 challenge thread.
PGP Key and corresponding e-mail address
|
|
|
| Pages:
1
2
3
4
5
6
..
22 |