| Pages:
1
2
3
4
5 |
LargeV
Harmless

Posts: 12
Registered: 28-11-2015
Location: Connecticut
Member Is Offline
Mood: No Mood
|
|
I dissolved 1.5g sodium bisulfate in 20 ml of water and added 10 ml of 3% hydrogen peroxide and then added a 1987 US penny.
I left it out for 6 hours and when I put in a few pellets sodium hydroxide, it made this weird olive yellow-green substance that went away when the
solution was swished.
I pippeted 1 ml of the solution into a solution with half a gram of sodium hydroxide, and it made a green precipitate/colloid.
This turned brown-black when heated, so I presume I accidentally made copper peroxide.
I then left it out for a day, and I come back to see that the penny has a huge black oxide layer and bubbles quite violently when I put another gram
of sodium bisulfate in.
Then, I put in another few pellets of sodium hydroxide. The sodium hydroxide pellets sit inside the solution for a second and start to grow a huge
white insoluble snake out the top of the pellets.
I thought this was weird, so I pipetted 1.5 ml in the sodium hydroxide solution with the precipitate of copper peroxide I left out yesterday, and it
dissolved the copper peroxide, which I am pretty sure is insoluble.
What even?
http://i.imgur.com/fZre1F1.png http://i.imgur.com/4y7NNt2.png
[Edited on 11/28/2015 by LargeV]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Starting to wish that I never started this thread... It's turned into a string of unscientific and poorly executed experiments that all need to be
redone in a more controlled way.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cheer up !
LargeV just made it better.
What's in a 1987 US penny ?
Copper mostly i guess.
[Edited on 29-11-2015 by aga]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
US pennies made after 1983 have been made of zinc plated with copper. So it's quite likely that there is some zinc contamination present. I'd
recommend using copper wire as a source for copper as it needs to be quite high purity to function properly. Copper pipe is decent, but not quite as
good. Pennies, even the pre-1983 ones, will contain zinc. Though often cited as being pure copper, pre-1983 pennies also contain 5% zinc.
Perhaps try again with pure copper wire.
|
|
|
LargeV
Harmless

Posts: 12
Registered: 28-11-2015
Location: Connecticut
Member Is Offline
Mood: No Mood
|
|
I will try to find some! Thank you for the feedback 
|
|
|
LargeV
Harmless

Posts: 12
Registered: 28-11-2015
Location: Connecticut
Member Is Offline
Mood: No Mood
|
|
I have a suggestion of what this mystery snake actually is. Because of the zinc contamination, it might have formed zinc hydroxide, which is white and
insoluble like the snake! I will report back on my results with pure copper.
EDIT: I have my results! It was, in fact, the zinc! I tried with a piece of pure copper metal, it dissolved to form copper sulfate which I did not see
in the first trial.
I then tried it with a 1972 penny which also did form copper sulfate without the zinc contamination too, as evidenced by only copper peroxide forming
on addition of the hydroxide!
[Edited on 11/29/2015 by LargeV]
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
I prepared fresh Cr2O3 by thermally decomposing ammonium dichromate. This Cr2O3, I tried to dissolve in 25% HCl, wanting to prepare CrCl3. The
solution came out yellow. It does not change its color when I add strong acids (H2SO4 conc) or strong bases (NaOH). It, however, does change color to
very pale green when I add a reducer such as sodium thiosulfate.
What is the yellow thing? Is it some complex of CrCl3, or hexavalent chromium?
Addendum: adding very strong NaOH resulted in the solution changing color to pale green and a wispy precipitation forming.
Addendum 2: I added potassium permanganate to the yellow liquid, and it turned deep brown, almost opaque, but with no precititate. This brown liquid
was acidic and very bleaching, it discolored the paper strip after turning it red, and smelled of chlorine. But no massive evolution of chlorine.
Addendum 3. Re-did the experiment with a mixture of sulfuric and hydrochloric acids dissolving Cr2O3. Situation normal, a green solution which, I
think, is a mixture of chromium chloride and sulfate. Addition of KMnO4 results in massive evolution of chlorine.
[Edited on 29-11-2015 by ave369]
Smells like ammonia....
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
@ave369: Did the Cr2O3 dissolve completely in the HCl? If not, then my guess is there was still some ammonium
chromate/dichromate left in your chromium(III) oxide, which simply leached out in the HCl.
I just tested a dilute, yellowish, potassium dichromate solution (I have no ammonium dichromate): concentrated sulfuric acid turned it slightly more
orange (though still yellow), while a large amount of sodium hydroxide turned it greenish.
[This was posted after Addendum 2 & before Addendum 3.]
[Edited on 29-11-2015 by MolecularWorld]
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
I'll repeat the experiment with a specimen of well leached Cr2O3 I have.
Smells like ammonia....
|
|
|
LargeV
Harmless

Posts: 12
Registered: 28-11-2015
Location: Connecticut
Member Is Offline
Mood: No Mood
|
|
I tried making copper peroxide in dilute solutions, and it made this orange precipitate with no signs of copper hydroxide.
0.5g CuSO4 + 3ml H2O2 + 10ml water + 1.5ml 0.5M NaOH
[Edited on 12/4/2015 by LargeV]
|
|
|
MolecularWorld
Hazard to Others
  
Posts: 110
Registered: 30-10-2015
Member Is Offline
Mood: No Mood
|
|
That's similar to a reaction aga documented upthread.
Copper peroxide is only stable below 6*C, above which it decomposes to other copper oxides and oxygen; the orange is probably a finely divided
CuO/Cu2O precipitate.
[Edited on 4-12-2015 by MolecularWorld]
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
(If you all don't mind)Back to my previous experiments:
I've obtained more NH4OH, so I may repeat some of my previous experiments, and perhaps make some more accurate observations.
Meanwhile, I discovered something while reading the Wikipedia article of NH4OH:
| Quote: |
When ammonium hydroxide is mixed with dilute hydrogen peroxide in the presence of a metal ion, such as Cu2+, the peroxide will undergo rapid
decomposition.
|
Which explains partially of what I observed, and corroborates what @MolecularWorld was hypothesizing. Hm, I should have done some more research, I
admit.
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
Aurium
Harmless

Posts: 46
Registered: 4-10-2015
Member Is Offline
Mood: Energetic
|
|
Hi guys,
So I tried to make some nitrocellulose. I had made it many times before with success.
This time however I tried using cotton string, expecting to end up with string form NC.
Regular H2SO4 + KNO3 + HNO3 nitration bath. I cannot remember the exact amounts I used right now. I made some calculations conserving pH and [NO3],
anyway,
This happened:
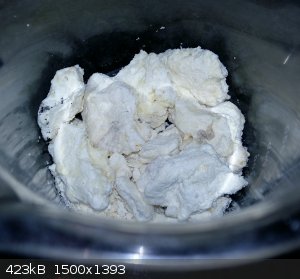  
So I added the string unrolled. Immediately after hitting the acid it curled up into a big ball.
I finished the nitration as best I could and washed it neutral. I then broke it into smaller pieces.
Now, this thing, plastic like nitrocellulose:
- Not ignitable at all. Just decomposes over flame.
- Not soluble in Acetone. At all, not soluble in dry boiling acetone whatsoever.
- Is quite rigid.
I am aware that NC was used a common plastic in older days, but I find it strange that this isn't soluble in acetone.
Some insight as to why my NC turned out so different?
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
It's not what I made; it's what did he make:
https://m.youtube.com/watch?v=DJGVZNth68k
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
There is a thread on this from about a year ago. Look up "transparent sodium". Also look at some related YT vids by thunderf00t. He does the same
with potassium.
The transparent bead is not sodium. Best guesses are that it is Na2O or NaOH or some combinations thereof. But there are a whole bunch of unanswered
questions:
What is the blue/black coating that appears between the flame and the transparent bead?
What exactly is the paper doing to cause this effect? (I have done some experimenting and found that with Na, some contact with the paper is
necessary -- it is not merely a matter of limited availability to water. K behaves differently and will give the effect without paper.)
Why does the bead crackle and explode at the end?
If the bead is solid NaOH or Na2O, why does it not sink in the water.
There is a lot going on. Given that the actual mechanism for Na explosion in water was not determined properly until about 18 months ago (Coulombic
explosion), I think it will take some time for all of these questions to be completely resolved. Nevertheless, if you have some Na then it is worth
having a play with it. I for one would prefer to see a bunch of YT clips investigating this than repeated clips of hoons chucking sodium or potassium
into ponds.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
The tetramine copper sulfate reaction with hydrogen peroxide forms copper peroxide
Check my link in my signature I have pictures of what is described
[Edited on 24-8-2017 by symboom]
[Edited on 24-8-2017 by symboom]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I've noticed that when I dissolve nickel or copper using HCl and peroxide, that it eventually starts to smell different, and actually a bit...
pleasant. This isn't really a word I'm used to using to describe compounds made exclusively of strong oxidizers. My best guess is that it's
perchloric acid, since it doesn't smell like chlorine or a strong acid. Although this isn't among the various methods that are described to
synthesize it, I'd guess that the metal being dissolved works as a catalyst, because nickel and copper catalyze so many other reactions.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by LargeV  | I have a suggestion of what this mystery snake actually is. Because of the zinc contamination, it might have formed zinc hydroxide, which is white and
insoluble like the snake! I will report back on my results with pure copper.
EDIT: I have my results! It was, in fact, the zinc! I tried with a piece of pure copper metal, it dissolved to form copper sulfate which I did not see
in the first trial.
I then tried it with a 1972 penny which also did form copper sulfate without the zinc contamination too, as evidenced by only copper peroxide forming
on addition of the hydroxide!
[Edited on 11/29/2015 by LargeV] |
Close! You formed sodium zincate.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
myristicinaldehyde
Hazard to Others
  
Posts: 166
Registered: 23-4-2016
Location: .͐͌ ͛҉̻̫̰̻̖E̮ͮ̐́̚ ̢̗̅̉ͩ͂̒̌.̯̻̺̯̀̎͂̄ͩ̚
Member Is Offline
Mood: сорок пять
|
|
I have something mildly interesting.
A solution of copper sulfate and dilute sulfuric acid, when left with tin pellets, plates the pellets with copper. However, the supernatant, after
dropping a precipitate of tin oxide/hydroxide/oxysulfate is a clear green, like the tetrachlorocuprate complex, but maybe slightly more blue.
Why isn't the solution the gentian blue of hexaaquacopper(ii)? (My best guess is the presence of cu(i) ions, but they are often white and insoluble,
which doesn't explain a clear green solution)
If we don't study the mistakes of the future we're doomed to repeat them for the first time.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I came up with a weird one today.
Some background -- Following a textbook procedure to determine the empirical formula of copper oxide -- teaching students about gravimetric analysis.
Procedure called for CuO to be dissolved in sulfuric acid. Then zinc added to precipitate out the copper quantitatively. Then washing, drying and
weighing to determine the mass of Cu in the original sample.
We did not follow procedure exactly. In an effort to accelerate the dissolving process we used a decent excess of concentrated sulfuric acid (the
"add a splash" method.)
This led to an overly acidic solution and hence the zinc granules added reacted producing H2.
Feeling a need for excess zinc I finished off the bottle. It was reagent grade but who knows its history.
The result was a yellowish solution and a grey black sediment.
The sediment showed traces of copper in a flame test but not much. It resisted dissolving in either HCl or H2SO4.
The yellow solution showed no indication of copper present. It had trace amounts of Fe3+.
Curious as to what may have caused the yellow tinge one of the students suggested a lead contaminant. We added some potassium iodide to check this
but did not get the instantaneous vivid yellow that one would expect if lead was present.
What we did get was a slowly-forming orange precipitate (photographed). The colour was reminiscent of cadmium compounds and we discussed the
likelihood of this. It was a nice idea but I think can be dismissed since the colour does not match CdI2 or CdSO4. CuI2 is not a match either.
So, I am curious. What the hell did we just make?

[edit]
The colour is brighter than the photo indicates. Similar I guess to the colour of potassium dichromate.
[Edited on 25-10-2017 by j_sum1]
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
Could it be precipitated Cu2O (copper(I) oxide)? The color looks a bit too fluorescent to be Cu2O in my opinion but the colors are pretty close.
The philosophy of one century is the common sense of the next.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by ninhydric1  | | Could it be precipitated Cu2O (copper(I) oxide)? The color looks a bit too fluorescent to be Cu2O in my opinion but the colors are pretty close.
|
I confess to not being too familiar with copper (I). But that idea had occurred to me. It (my mystery compound) is very orange and bright. I am not
sure why the oxide would precipitate after the addition of iodide. And I would have thought that either of the two copper oxides would be
incompatible with conditions that are still highly acidic.
FWIW, a spritz of the solution through a bunsen flame did not reveal any green or blue coloration. And there was no visible reaction with
concentrated ammonia. I guess that only rules out Cu(II).
I might add some peroxide to the yellow solution and see if any blueness appears.
[Edit] Clarification and addendum.
And no visible reaction attempting to oxidise any Cu(I) to Cu(II). There did seem to be a temperature rise though.
[Edited on 25-10-2017 by j_sum1]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I bet it's a sulfide of some sort. Zinc will reduce sulfuric acid to zinc sulfide, especially in a concentrated solution, then hydrolyze to ZnO and
H2S. H2S would react quickly with any copper salts, precipitating CuS, which is black. Elemental sulfur could be responsible for some of the yellow
color, or there could be any of a number of mixed sulfides.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Melgar  | | I bet it's a sulfide of some sort. Zinc will reduce sulfuric acid to zinc sulfide, especially in a concentrated solution, then hydrolyze to ZnO and
H2S. H2S would react quickly with any copper salts, precipitating CuS, which is black. Elemental sulfur could be responsible for some of the yellow
color, or there could be any of a number of mixed sulfides. |
That sounds like a bit of a stretch. CuS is very
black indeed, just as dark as CuO, and if it was in there, you'd be looking at a muddy brown color, not a bright orange.
Edit: Also interesting to look back at when I started this thread in my early days. My original puzzle of a red manganese solution was most certainly
just manganate disproportionating into permanganate and very finely divided manganese dioxide. I couldn't believe at the time though that a solution
could contain a precipitate while still appearing completely transparent, and it wasn't until later experiments with Prussian blue that I came to
realize that.
[Edited on 10-25-2017 by zts16]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by zts16  | | That sounds like a bit of a stretch. CuS is very black indeed, just as dark as CuO, and if it was in there, you'd be looking at a muddy brown color,
not a bright orange. |
The precipitate was described as being black, although the orange color is almost certainly from iodine. I missed that part the first time around.
Add sulfuric acid to a potassium iodide solution, and see what color it turns. No metals necessary.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
| Pages:
1
2
3
4
5 |