| Pages:
1
2
3
4
5 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
catechol
I remember reading on this forum about making catechol using calcium bromide. This was 1-2 months ago (I think). But I can't find anything on this.
I even bought 25g of Ca(Br)2 with which to make it.
Does anybody remember this synthesis?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
From phenol?
Phlogiston manufacturer/supplier.
For all your phlogiston needs.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
yes, I believe it was! It might have been in a referenced paper.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
It's entirely possible to transform phenol into catechol using hydrogen peroxide. I don't know what catalyst is typically used, but it could be a
halide.
Phlogiston manufacturer/supplier.
For all your phlogiston needs.
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
https://www.google.ie/search?q=catechol+from+phenol+site%3Aw...
One up from the bottom
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thanks so much yobbo II. I have a 400w source of h√ so will give this a try.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I think I have found 2 better syntheses for catechol:
One starts with guaiacol and uses 48% HBr. This is a 1941 synthesis by Taylor & Clark published in OrgSyn.
The other starts with salicylaldehyde and uses HOOH as reagent. By-product is the formate ion. This is by Dakin and is also published in OrgSyn
(1941).
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
I would advise strongly against the Dakin reaction, the yields tend to suck balls, also you would first have to either prepare of get your hands on
salicylaldehyde.
http://www.nrcresearchpress.com/doi/pdf/10.1139/v73-357
I have however prepared 2-aminophenol by hoffman rearangement of salicylamide followed by hydrolosis of the resulting benzoxazolone.
Unfortunately i still haven't gotten round to performing the sandmeyer reaction as getting my hands on sodium nitrite in NZ is an absolute pain in the
buttox.
I followed the following prep and got close to 80% yield starting from salycilic acid, if i recall major loss was during the hoffman rearrangement.
I had an issue doing a large scale batch however (30g), during hydrolysis of the benzoxazolone i foolishly used too high of a concentration of HCl
which lead to it not working at all.
A second attempt with dilute HCl worked however it was very slow. I do not know why this requires dilute HCl, if someone could enlighten me it would
be much appreciated.
http://www.sciencemadness.org/talk/viewthread.php?tid=63327#...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
OK thank you.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Magpie, its hard to go wrong with an OrgSyn procedure. I'd say pick whichever one is most accessible to you.
|
|
|
Swinfi2
Hazard to Others
  
Posts: 131
Registered: 19-2-2018
Location: England
Member Is Offline
Mood: Catalytic
|
|
Synthesis of catechol recently came up in my BSc research project, i found the Dakin oxidation and another method starting with Phenol that may be of
interest for making substituted catechols depending reagent availability. https://doi.org/10.1021/ol017068j
[Edited on 19-2-2018 by Swinfi2]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have on hand 50mL of guaiacol with which I plan to make catechol according to the OrgSyn procedure at 1/10 scale.
I began setting up the apparatus 3 days ago trying to assemble a workable assembly from an assortment of RBFs, condensers, etc. I then gave up and
decided to order the appropriate glassware. For one, I will use a 500mL vertical 3-neck RBF, 24/40, from eBay.
The separator was the main problem. I tried to use my Dean-Stark but it was a mess. So, I have designed my own separator and am now taking bids on its
construction from two glassblowers.
Fortunately, I have a 600mm Hempel column, 19/22. This should do nicely for the requirement of at least a 400mm column.
I apologize for the drawing being upside down. Toggle it; it will right itself.
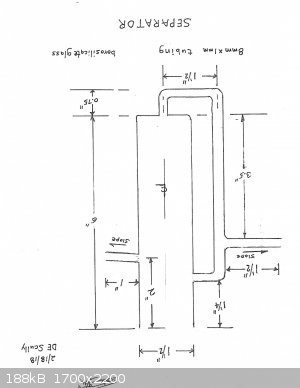
[Edited on 19-2-2018 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Hydroxylation and decarboxylation of hydroxybenzoic acids by
Fe2+-chelates
Halvor Aarnes, Department of Biology, University of Oslo, POB 1066, Blindern, N-0316 Oslo,
NORWAY; E-mail: halvor.aarnes[at]bio.uio.no
Unpublished research paper based on experiments done in 1998-1999.
Abstract
Hydroxylation and decarboxylation of hydroxybenzoic acids occurs rapidly at pH 3 to 6.5 in a system
con 4 2 taining FeSO and Na EDTA. EDTA could be replaced by citric acid. In this in vitro system 4-
hydroxybenzoic acid is hydroxylated to 3,4-dihydroxybenzoic acid (protocatechuic acid) and
decarboxylated to hydroquinone. In an analogous reaction 2-hydroxybenzoic acid (salicylic acid) is
hydroxylated to 2,3-dihydroxybenzoic acid and 2,5-dihydroxybenzoic acid (gentisic acid) and
decarboxylated to catechol. Surprisingly the reactions showed Micahelis-Menten saturation kinetics for
the products, and this paper is the first description of these reactions. From these data it is also
cautioned to use hydroxylation of hydroxybenzoic acids as an indicator of oxidative stress and hydroxyl
radicals in biological systems without proper controls.
Attachment: Hydroxylation and Decarboxylation of hydroxybenzoic acids by Fe2+-chelates.pdf (884kB)
This file has been downloaded 458 times
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
An interesting paper, though it leaves some questions unanswered. Is the iron catalytic, or does it need to be used stoichiometrically? If the
reaction is allowed to run longer, will it eventually fully decarboxylate all of the benzoic acids present? In the paper, they say they only got 13%
yield of catechol from salicylic acid, with 50% 2,5-dihydroxybenzoic acid and 37% 2,3-dihydroxybenzoic acid. Sure it would be easy to separate the
catechol from the acids by neutralizing the acids with a weak base and extracting, but it would be nice if you could just convert everything into
catechol.
I'm filing this in the "stuff I'd like to try when I actually have lab time" folder 
Edit: Merged three catechol preparation threads into one big happy catechol thread
[Edited on 2-20-2018 by Texium (zts16)]
|
|
|
Texium
|
Threads Merged
20-2-2018 at 07:05 |
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | I would advise strongly against the Dakin reaction, the yields tend to suck balls |
While I agree with your
complaint about salicylaldehyde, this is not supported by your link, in which the highest yield of catechol is 89%. It appears that the key is to use
reagents at a very low concentration. This accords with the famous Orgsyn procedure in which the yields are decent and 3% off-the-shelf peroxide is
used:
http://orgsyn.org/demo.aspx?prep=cv1p0149
[Edited on 20-2-2018 by clearly_not_atara]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Why not protocatechuic acid from alkali fusion of vanillin or eugenol, and distillation?
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
If you're going to use a demethylation of guaiacol, why not start from the considerably more available guaifenesin, readily purified from OTC
expectorants.
To reduce the consumption of HBr, I'm under the impression that treatment of guaifenesin with permaganate produces (2-methoxy-phenoxy)acetic acid.
|
|
|
Swinfi2
Hazard to Others
  
Posts: 131
Registered: 19-2-2018
Location: England
Member Is Offline
Mood: Catalytic
|
|
I've been creating catechol complexes in my uni lab. So far I've found oxygen seems to oxidise/cleave the catechol ligands of complexes but it only
seems to occur in solution, the things aren't super sensitive (except the chrome complex which is very sensitive) but i think its worth knowing if you
leave a solution instead of a dry product it might not be what you want when you come back to it.
Hope this is helpful guys 
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by UC235  | If you're going to use a demethylation of guaiacol, why not start from the considerably more available guaifenesin, readily purified from OTC
expectorants.
To reduce the consumption of HBr, I'm under the impression that treatment of guaifenesin with permaganate produces (2-methoxy-phenoxy)acetic acid.
|
I've looked into using OTC guaifenesin as a building block and it's very expensive for the amounts you can
recover from the tablets, even assuming 100% recovery. You'd be better off buying catechol online.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Huh?
Thirty-odd dollars per pound, is too expensive?
https://www.amazon.com/Guaifenesin-USP-Powder-Bulk-Pound/dp/...
Never hurts to check around.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The point is to make reagents not buy them, at least the first time. This way you learn some chemistry.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Mea Culpa!
I'm good with making reagents, if they are very expensive, or can't be bought easily.
And, these days, most things are very expensive, or can't be bought easily.
So..... When I see something that isn't outlandishly priced, and I CAN buy it easily, I am sorely tempted.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
OTC guaifenasin. As in, Mucinex. Which comes in tablets, which must be
purified, and is much more expensive by mass. Besides, I've seen catechol at $46/pound which when you factor in the lower molecular weight of
catechol, is a much better deal: https://www.adorama.com/pyct1lb.html?gclid=CjwKCAiA_c7UBRAjE...
I understand you want to make it yourself for the experience, Magpie. I'm mainly posting this info in case anyone is just interested in trying to
source catechol in bulk for other reactions. Also, I'm curious: where did you get your guaiacol? It seems to be more expensive and less available than
catechol, so I find it odd that you'd be going in that direction, rather than the other way around.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I paid $15.95 for 50 mL of USP guaiacol. Source: www.medlabsupply.com
[Edited on 27-2-2018 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Magpie  |
The separator was the main problem. I tried to use my Dean-Stark but it was a mess. So, I have designed my own separator and am now taking bids on its
construction from two glassblowers.
|
My 1st choice glassblower (National Scientific) was too busy to give me a bid, so he recommended HS Martin, a vendor with decades of experience who
has a good reputation. But my 2nd choice was Eagle Laboratory Glassblowing. HSMartin's bid was $250, Eagle's was $75. Eagle gets the job.
[Edited on 27-2-2018 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
| Pages:
1
2
3
4
5 |