| Pages:
1
2
3
4
5 |
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
lol
I would not call it an apparatus. It was a temporary thing.
Yes, the bromine will come out hot therefore you need to have a long glass tube onto which you can apply some ice water in order to get some good
condensation. I would personally use salt water with a temperature of -15 °C. It would cause some of the bromine to solidify/half liquid.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
In that case, why wouldn't the collection flask be in a bath of icy salt water? The bromine would partially freeze, and that would get rid of some of
the dangers of bromine. (Slushie of death.... MWAHAHAHA)
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I just found a way to oxidize pool 30% NaBr to bromine easily, fast and without much smell
I was trying the oxidation of NaBr using nitric acid, I add added enough sulphuric acid to NaBr sol. to convert it all to HBr, then I added some HNO3
70%, nothing happened, even after few minutes, so I added about 0.2g of NaNO2, vigorous effervescence occurred, and within second, a pool of bromine
was formed on the bottom.
15 ml 30% Bromide
5 ml 70% HNO3
5 ml 98% H2SO4
0.2g NaNO2
I need to work to make the reaction perfect for preparative scale.
[Edited on 16-5-2013 by plante1999]
I never asked for this.
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
I'd like to try that out but what do you mean 15ml 30% bromide? Is your bromide in solution already when you buy it or do you have a specific weight
on hand?
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
It comes on solution already for pool, don't forget to mix well, and if the solution only become bright red, add more HNO3.
I never asked for this.
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Are you sure that is exactly what you did? I duplicated this but on a 5x smaller scale. I didnt need nitrite, After the first drop of HNO3 it started
vigorously bubbling and I mean VIGOROUSLY just like plante said! Spewing bromine vapor everywhere, and the liquid almost boiling out, I had to vacate
the garage. In the end I did have a small amount of bromine that sunk to the bottom but I had to have lost 90% during the eruption. Like you said
though the prep needs a little work lol.
-EDIT-
This pic is after I added water to calm everything down, only a couple drops of actual bromine at the bottom.
I think with some tweaking it will be a fast way to get low yield Br2.

[Edited on 5-16-2013 by chemcam]
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have done a lot of personal research on this reaction and I have found that if you have very pure bromide with very pure nitric acid, then no
reaction occurs. As soon as a little NO2 is added, then suddenly the nitric acid oxidizes the bromide to bromine. Adding NO2 can be done by adding a
pinch of NaNO2 (which decomposes in acid), but adding another reductor also helps (e.g. some copper metal, or some Na2SO3). The reductor reacts with
the nitric acid and this gives some NO+NO2 and then the reaction with bromide sets off.
This is not a good way to make bromine. The bromine will be highly contaminated with ONBr. ONBr is a very dark liquid and it mixes well with bromine.
Gaseous ONBr is brown, more chocolate brown than red-brown, but a mix of ONBr and Br2 is very difficult to distinguish from pure bromine, especially
when viewed just by eye. An interesting experiment though may be Endimion17's infrared imaging of bromine, which contains a lot of ONBr. That would be
a nice way of testing the purity of bromine, produced in this way (provided that ONBr is not 'colorless' in IR).
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
I just might be able to do that, microscale. That's about the largest scale I can do, given the quantities of bromide salts I currently have.
Therefore, distillation is out of the question.
Write a short, simple procedure and I'll see what I can do. You can expect results next week.
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Take a few ml of concentrated nitric acid and put this in a test tube. Make a picture of this with your camera. Just to check whether nitric acid is
'colorless' in infrared as well as in visible light.
Add a ml of concentrated solution of NaBr or KBr (you could also make a picture of this before you add it to the nitric acid).
Add a pinch of NaNO2 or KNO2 and let the reaction run. If you don't have NaNO2 or KNO2 you could try to add some Na2SO3 or any other suitable
colorless reductor which gives NO2 with nitric acid. Try to keep as much of the bromine in the test tube, such that a decent blob of liquid remains at
the bottom of the test tube.
Make a picture after the reaction has completed. I am looking forward to your results.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
From a very distant past I seem to remember that adding KI solution to a solution of CuSO4 gives a redox reaction, releasing iodine that turns the
solution brown. Addition of CCl4 and shaking a bit, indeed gave a purple drop of iodine dissolved in the CCl4 at the bottom of the testtube.
Is this a known reaction, or was there an issue with the purity of the chemicals? If it works with iodine, might it work with bromine too?
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Copper II iodide is unstable, and disproportionnate to copper I and iodine, the bromide does not do that. It seac acid and it seams my nitric acid and
bromide is quite pure then. Glad to ear that, my reaction was slow, and after NaNO2 addition some bubling was observed, but nothing very fast, the
solution turned brown, then clear and a pool of bromine was formed.
With 120ml of 30% bromide I had got about 5-7ml of bromine.
I never asked for this.
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Copper(II) iodide does not really disproportionate, it simply does not exist.
What you describe is a simple redox reaction:
2Cu(2+) + 2I(-) --> 2Cu(+) + I2
In the presence of excess iodide ion, a precipitate is formed as well:
Cu(+) + I(-) --> CuI
So, if you add copper(II) and iodide to each other you get a precipitate of CuI (which is off-white) and you get iodine.
Copper(II) is not a sufficiently strong oxidizer to oxidize bromide ion, so nothing similar happens with bromide.
@plante1999: The word 'disproportionate' has the meaning that one compound shows a redox reaction with itself, where one part acts as oxidizer and the
other part acts as reductor.
A well-known example is 2Cu(+) --> Cu(2+) + Cu (disproportionation from +1 to +2 and 0)
Another well-known example is 3BrO(-) --> 2Br(-) + BrO3(-) (disproportionation from +1 to -1 and +5)
The reverse also exists. That is called comproportionation. In that case two compounds with the same element in different oxidation states react to
form a single compound with oxidation state somewhere between that of the original reactants. An example is the reaction of bromate and bromide to
elemental bromine in acidic solution (comproportionation from +5 and -1 to 0).
Whether disproportionation or comproportionation occurs depends on the redox potential for transfer from oxidation state to another. A Frost-diagram
nicely shows whether this occurs or not (please use Google to find more about that subject).
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
I know what disproportionate mean...
Chlorine in NaOH, etc...
Simply that I messed up a little what I meant,. I always taugth copper II iodide was simply way too instable, at least thats what old chemistry books
says. Iodide in copper II iodide is reductor, and copper II the oxidizer, in this way it is a disproportion reaction.
I never asked for this.
|
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
Quote: Originally posted by woelen  | | I have done a lot of personal research on this reaction and I have found that if you have very pure bromide with very pure nitric acid, then no
reaction occurs. As soon as a little NO2 is added, then suddenly the nitric acid oxidizes the bromide to bromine. (cut) |
My acids are ACS grade but I used pool grade 99% bromide. I do have ACS grade bromide though, but only 125g so I was saving it, I will use that later
and record it.
Quote: Originally posted by woelen  | Take a few ml of concentrated nitric acid and put this in a test tube. (cut)
Add a ml of concentrated solution of NaBr or KBr (you could also make a picture of this before you add it to the nitric acid). (cut)
|
I am going to perform this in a couple hours or so and I will record a video. I notice though that you don't mention H2SO4 in this prep. Was it
overkill that I used HNO3 and H2SO4 in my last run, could it have cause all the spewing? I imagine it got fairly hot. I don't have infrared on my
camera but i'll show the reagents I used to prove purity.
[Edited on 5-16-2013 by chemcam]
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@plante1999: Disproportionation is specific to a single element in a single compound to act as oxidizer and at the same time as reductor. So, Cl2
reacting to give chloride and hypochlorite is indeed disproportionation, but CuI2 decomposing to CuI and I2 is not disproportionation, it is a normal
redox reaction, in which copper(II) is the oxidizer and iodide is the reductor.
@chemcam: It is the IR-part which I find most interesting, but of course, it is always nice to have other people perform the reaction. The presence of
conc. H2SO4 certainly will add to the reactivity of the mix. It makes the nitric acid (more) anhydrous and this makes the acid much more reactive and
may even lead to decomposition of some HNO3 with formation of NO and/or NO2, which could be the reason for the violent reaction in your case.
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Sorry for disproportion misconception, but I'm sure I read something about CuI2 in a old book. As for H2SO4, my idea was to save on nitric acid, as
the bromide need to be turned to hydrobromic acid for the reaction, nitric acid do the trick too, but for me, it is way more costly.
[Edited on 16-5-2013 by plante1999]
I never asked for this.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
What if you just leave the test tube in an ice bath before adding the NaNO2? This would keep the temperature under control.
NOBr undergoes photolysis or something like that. Maybe flashing a really strong light from different points might help getting as much NOBr as you
can out of the Br2.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
chemcam
Hazard to Others
  
Posts: 423
Registered: 18-2-2013
Location: Atlantis
Member Is Offline
Mood: I will be gone until mid-september, on a work contract.
|
|
You guys were right, when I used my high grade sodium bromide, NaNO2 was required to get the reaction going.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
woelen, I'm sorry for the delay. Here are the results of the NIR camcorder imaging.
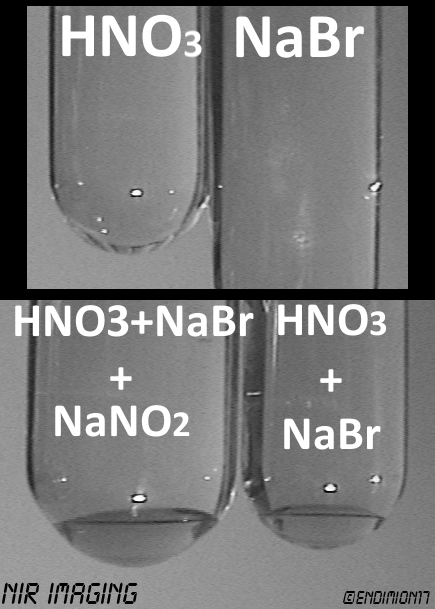
No nitrites are required for the initialization of the reaction. After you mix the solution of bromide ions with nitric acid, it takes a couple of
minutes for the reaction to start. At first it really doesn't look like anything is happening, but you have to wait.
It is not violent. The solution turns yellow, then brownish, and then turbid brown. Bromine starts raining down, precipitating from a "cloud".
Business as usual.
It obviously depends on the temperature and the concentration of reactants.
The NIR images were taken few days after the reactions were complete. In VIS, both solutions look like Coke, with the exception of the blob of bromine
at the bottom, which is pitch black.
Nitrites kind of speed things up. I think more heat was given off, too, and the colour change was faster.
Maybe they're consumed, maybe it's catalysis, I don't know and won't even bother. This is a messy, expensive and hazardous way to oxidize the
Br<sup>-</sup> anion.
[Edited on 4-6-2013 by Endimion17]
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Thanks for doing this experiment. Unfortunately it is not conclusive about impurities in the bromine. There are two possibilities:
1) The impurities are 'colorless' in IR light.
2) There are no impurities, other than water and acid.
Could you try one other experiment? The experiment is fast and very simple and produces a lot of ONBr, one of the supposed contaminants in the
production of Br2 from NaBr and HNO3.
The experiment goes as follows:
- Prepare a very concentrated solution of NaBr in water.
- Add approximately an equal volume of 50% H2SO4 (not nitric acid). Some crystals of NaHSO4 may form, just decant the liquid from these crystals and
keep the liquid.
- Add some solid NaNO2 to the liquid.
If you have HBr, then things are even more simple. Just add some solid NaNO2 to 40% HBr.
You get a very dark liquid (nearly black) and a chocolate brown gas. This gas is ONBr, not Br2. It is very dense (you can pour it out easily). The
solution in water is practically black. If the liquid is diluted, then the ONBr hydrolyses and the liquid becomes colorless or yellow, due to the
formation of a little amount of Br2 as well.
This is the gas and liquid which I really would like to see in IR imaging. If ONBr is 'colorless' in IR-light, then things still are not conclusive,
but if ONBr is dark in IR light as well, then your other experiment gives interesting information.
I really wish I could do this kind of experiments myself. I dare not take apart my 400 euro digital camera, however, for trying out the filter trick.
[Edited on 4-6-13 by woelen]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Picked up an old book yesterday at a thrift store. Feel like transcribing something. Enjoy: | Quote: | <div align="center"><strong>CHAPTER 18<br />The Chlorine
Family<br /><hr width="70" />BROMINE</strong></div> <strong>Preparation of bromine.</strong> The
laboratory method and the industrial method for preparing bromine are as follows:<br /> 1.
<strong><em>Laboratory method.</em></strong> Just as chlorine is set free by the oxidation of hydrochloric acid by manganese
dioxide (p. 203), so bromine may be prepared by a similar reaction, substituting hydrobromic acid for hydrochloric : <table style="float:
right"><tr><td> </td></tr></table><br /><br />4(H<sup>+</sup>, Br<sup>-</sup> </td></tr></table><br /><br />4(H<sup>+</sup>, Br<sup>-</sup> + Mn<sup>++++</sup>, 2 O<sup>--</sup> → Mn<sup>++</sup>, 2
Br<sup>-</sup> + 2 H<sub>2</sub>O + Br<sub>2</sub> ↑<br /><br />Hydrobromic acid is unstable
and on this account is not usually available in the laboratory ; so a mixture of sodium bromide and sulfuric acid is used instead. The equation for
the complete reaction is like the one for chlorine (p. 203) :<br /><br />2(Na<sup>+</sup>, Br<sup>-</sup> + Mn<sup>++++</sup>, 2 O<sup>--</sup> → Mn<sup>++</sup>, 2
Br<sup>-</sup> + 2 H<sub>2</sub>O + Br<sub>2</sub> ↑<br /><br />Hydrobromic acid is unstable
and on this account is not usually available in the laboratory ; so a mixture of sodium bromide and sulfuric acid is used instead. The equation for
the complete reaction is like the one for chlorine (p. 203) :<br /><br />2(Na<sup>+</sup>, Br<sup>-</sup> + 2(2 H<sup>+</sup>,
SO<sub>4</sub><sup>--</sup> + 2(2 H<sup>+</sup>,
SO<sub>4</sub><sup>--</sup> +
Mn<sup>++++</sup>, 2 O<sup>--</sup> → 2 Na<sup>+</sup>,
SO<sub>4</sub><sup>--</sup> + Mn<sup>++</sup>, SO<sub>4</sub><sup>--</sup> + 2
H<sub>2</sub>O + Br<sub>2</sub> ↑<br /><br
/> <strong>Laboratory apparatus.</strong> The materials are placed in a retort,
<em>A</em>, arranged as shown in Fig. 162. The delivery end of the retort dips slightly into the water in flask <em>B</em>,
which is partly immersed in ice water (<em>C</em> +
Mn<sup>++++</sup>, 2 O<sup>--</sup> → 2 Na<sup>+</sup>,
SO<sub>4</sub><sup>--</sup> + Mn<sup>++</sup>, SO<sub>4</sub><sup>--</sup> + 2
H<sub>2</sub>O + Br<sub>2</sub> ↑<br /><br
/> <strong>Laboratory apparatus.</strong> The materials are placed in a retort,
<em>A</em>, arranged as shown in Fig. 162. The delivery end of the retort dips slightly into the water in flask <em>B</em>,
which is partly immersed in ice water (<em>C</em> . As the contents of
the retort are headed, bromine distills over and is collected in the cold receiver.<br /><br />– <em>Introduction to
College Chemistry.</em> McPherson, Henderson, Fernelius, & Quill. Ginn and Company, 1942/46. (<a
href="http://catalog.hathitrust.org/Record/009227908" target="_blank">Hathi Trust</a> <img src="../scipics/_ext.png" /> . As the contents of
the retort are headed, bromine distills over and is collected in the cold receiver.<br /><br />– <em>Introduction to
College Chemistry.</em> McPherson, Henderson, Fernelius, & Quill. Ginn and Company, 1942/46. (<a
href="http://catalog.hathitrust.org/Record/009227908" target="_blank">Hathi Trust</a> <img src="../scipics/_ext.png" /> |
Perhaps a little easier to read:
<strong>4 HBr + MnO<sub>2</sub> → MnBr + 2 H<sub>2</sub>O + Br<sub>2</sub>
↑</strong>
<strong>2 NaBr + 2 H<sub>2</sub>SO<sub>4</sub> + MnO<sub>2</sub> →
Na<sub>2</sub>SO<sub>4</sub> + MnSO<sub>4</sub> + 2 H<sub>2</sub>O + Br<sub>2</sub>
↑</strong>
[Edited on 3.11.13 by bfesser]
|
|
|
bfesser
|
Thread Pruned
2-11-2013 at 15:07 |
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
A few notes from my most recent attempts:
-If the solution of bromine still looks red or even orange after distillation has supposedly 'ended' (water was coming over instead of bromine),
distill it again. This roughly doubled my yield.
-Do NOT use a Kjehldahl bulb - the bromine gas hung around in the bulb forever, being heavier than air, and never distilled over at all. I was only
able to recover it by disconnecting the setup and quickly attaching a flask full of sulfuric acid to one end and stoppering the other.
The original solution is still pale orange, so I'm going to try one more time and see what happens. Probably not much.
Right now, from 25g of sodium bromide, electrolysis at 2A for about 13 hours, and far too much sulfuric acid, it looks like I have about 1/3 of a
milliter of bromine (considering the density of bromine of about 3.1 g/mL, this is just over 1g). Now, for ampouling, I'm going to redistill from the
sulfuric acid I kept it in. My only worry is that if my condenser takes up so much volume, the bromine might well just evaporate to fill the space and
I'll lose all my yield.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The problem with using such small quantities is that your mechanical losses (such as loss through vapor, filling up a flask) may bring down total
yield very much. Mechanical losses tend to be independent of the amount used and losing e.g. 1 gram from 2 grams or losing 1 gram from 10 grams is a
lot of a difference.
If you use ordinary glassware for your distillation (e.g. 100 or 250 ml flasks, a cooler of 40 cm length, NS24 or NS29 joints), then using only 25
grams leads to near total loss. For this purpose I also purchased a micro distillation set with 25 and 50 ml flasks and NS14 joints.
|
|
|
grayson
Harmless

Posts: 2
Registered: 2-4-2017
Member Is Offline
Mood: No Mood
|
|
Sorry to revive this old thread, but I feel it worth mentioning that KMnO4 + NaBr + H2SO4 should be done in that order.
I had the acid and the NaBr reacted with excess acid. I was aware KMnO4 and H2SO4 create Mn2O7, and somewhat energetically, so I added the KMnO4 with
great caution.
It was... energetic.
A few grains make for a lot of sparks. Also bromine, but seriously do it the other way. Chilled with ice. Not salt ice, regular ice. Made that mistake
at one point too. Dry ice is right out.
Attachment: sparks.m4v (4.2MB)
This file has been downloaded 871 times
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Wow, that must have been a mess to clean up!
|
|
|
| Pages:
1
2
3
4
5 |