| Pages:
1
2
3
4
5
6
..
23 |
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Hah! I know how you feel, one time I read almost the whole DPPP thread... Only to be disappointing in the end by finding out it was in fact just AP!
 That's life. That's life.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Here's an energetic that has been under research in rocketry, as it produces high-performance, solid propellants, e.g. for this see EP0959058 Isp of
348.8 for HNF/HTPB/Al mixture (332.9 for AP counterpart). It also burns with smokeless combustion and is not hygrosopic. Some properties below, mainly
from Aerospace Propulsion Products B.V.:
Hydrazinium nitroformate
N2H5C(NO2)3 = CH5N5O6
mw: 183.09.
color: orange-yellow
Density: 1.872 g/cc.
Mp. 124 deg.
heat of formation: -17.2 kcal/mol.
Friction sensitivity: 25-36 N (RDX: 120 N).
Impact sensitivity: 2-4 Nm (RDX: 7.5 Nm).
UN test series III, thermal stability
test at 75 deg: passed.
Electrostatic discharge: 3-6 J (RDX: >4.5 J).
toxicity-LD50: 128 mg/kg (RDX: 100 mg/kg).
Though nothing about its explosive properties. Kamlet's method shows at 1.87 an estimate of 8261 m/sec close to as good as the exp. value of FOX-7
(8340m/s) and 36.4 GPa (estimated for FOX-7: 34.0; RDX: 34.6).
[Edited on 5-1-2009 by Formatik]
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Nitroformates are salts of trinitromethane. It is explosive by itself and forms explosive metal salts; energetic complexes such as
ethylenediamine-copper-nitroformate are also possible.
Definetely an interesting compound, but difficult to make. I searched the forums and didn't find report of a working synthesis 
Possible routes include
-reacting acetylene with fuming nitric acid in presence of Hg catalyst (nitrate is used comercially)
-react fuming nitric acid with isopropyl alcohol -> very bad yields; 150ml acid give ~10g if I remember correctly
-react silver nitrit with iodoform (I didn't find a detailed description)
-make tetranitromethane from fuming nitric acid and acetic anhydride; this can be reacted with alkali hydroxide to yield the salt of nitroform
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Coincidently I stumbled upon this paper and thought to post it in case anybody is interested:
Energetic Ethylene- and Propylene-Bridged Bis(nitroiminotetrazolate) Salts
Young-Hyuk Joo, Jean'ne M. Shreeve
Chemistry - A European Journal, 15 (2009) 3198-3203.
DOI: 10.1002/chem.200802326
Attachment: Energetic Ethylene- and Propylene-Bridged Bis(nitroiminotetrazolate) Salts.pdf (247kB)
This file has been downloaded 2219 times
|
|
|
TETS
Harmless

Posts: 2
Registered: 10-11-2009
Member Is Offline
Mood: No Mood
|
|
Today i was reading a russian book (via google translate),then i saw a strange composition which was R.D.X/tungsten 50:50.The interesting thing about
this stuff is very high value of Plate dent test,which is 8.09(compare with 7.35 for OCTOL).
Also it has very high D:2.9 and just medium VOD of 6500.These figures show that brisance actually related more to density than VOD IMO.
I hope this is right thread for posting that.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Quote: Originally posted by TETS  | | Today i was reading a russian book (via google translate),then i saw a strange composition which was R.D.X/tungsten 50:50. |
Hi TETS, yes, it's the right thread, and W/RDX is a strange comp..
Sintered tungsten has been used in S/C liners but RDX already has a low OB and PD tests tell little about explosive power.
Inerts reduce brisance but the hardness of tungsten increases the dent-effect. . .
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
Well in that case any hard metal should do the job to some extend.
How should i imagine this kind of composition?
A mean a fine powder mix would result in the metal being burned during detonation , right ?
Also larger particals would form (supersonic) flying bits of metal all over the place, making it a very unpleasant experience to be around.
Someone who can give some clarity ?
What a fine day for chemistry this is.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Quote: Originally posted by User  |
A mean a fine powder mix would result in the metal being burned during detonation , right ?
Also larger particals would form (supersonic) flying bits of metal all over the place, making it a very unpleasant experience to be around.
|
Larger particles would be pulverised on detonation but not oxidised!
As I said---the OB of RDX is on the low side for such a powerful HE.
Its detonation produces less smoke than, say, TNT, but it's still dark. . .
[Edited on 11-11-2009 by hissingnoise]
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
Thanks, I didnt take the oxygen balance in account, I guess i need glasses 
Well lets suppose it isnt capable of "burning" the metal particals.
Some kind of thermobaric effect would come into play I can imagine.
Due to the incredible heat produces by the detonation the particals probably burn up in the air unless they are big enough.
Does this text say anything about mesh size?
What a fine day for chemistry this is.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Any thermobaric effect would be very small since RDX, on detonation, produces a quantity of CO at very high temperature. . .
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Some metal powders can reduce the thermal stability of RDX; lead, e.g. has a significant effect in this regard. . .
|
|
|
argyrium
Hazard to Others
  
Posts: 123
Registered: 3-2-2008
Location: Pacific
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by TETS  | Today i was reading a russian book (via google translate),then i saw a strange composition which was R.D.X/tungsten 50:50.The interesting thing about
this stuff is very high value of Plate dent test,which is 8.09(compare with 7.35 for OCTOL).
Also it has very high D:2.9 and just medium VOD of 6500.These figures show that brisance actually related more to density than VOD IMO.
I hope this is right thread for posting that.
|
I believe these and similar formulations are also know as Dense Inert Metal Explosives (DIME). Read http://tinyurl.com/y8cojx
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Yes, I'd forgotten about them---they were in the news after the Gaza 'adventure', but then, M/A is neccessarily a very nasty business. . .
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I remember reading about this composition (50:50 RDX:W). IIRC, the authors were of the opinion that the abnormally deep dents were due to abrasion. If
that is indeed the case, it probably isn't a very useful effect.
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
A bit OT but I can safely and confidently tell you that a mix of Hydrazine Azide, Hydrazine Perchlorate and PETN, mixed to OB neutral, makes a VERY
good HE for chaped chardes, however it sucks up moisture like mad so it has to be sealed damn well. I left s 2" CSC for 6 months and she still fired,
penetrating a 4" RHS plate and punching through the 2" wooden backing - the only target I had on hand at the time. I tamped the SC after sealing it to
the target with a cornstarch/water thixotropic mix in a 5gal bucket which was also glued to the target. Ignition was electric, rear-center priming
with a CLD (A variant on Microteks design) and a 5g booster of cast PETN:ETN mix ala Rosco Bodine.
I see a beautiful future for N2H4 based material 
I reckon the plate dent with W added to RDX is doe to the Abrasion as said by Microtek, and due to very dense hot W particles applying a lot of KE to
the plate when 'pushed' by the RDX. Or maybe the W burns...
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by -=HeX=-  | A bit OT but I can safely and confidently tell you that a mix of Hydrazine Azide, Hydrazine Perchlorate and PETN, mixed to OB neutral, makes a VERY
good HE for chaped chardes, however it sucks up moisture like mad so it has to be sealed damn well. I left s 2" CSC for 6 months and she still fired,
penetrating a 4" RHS plate and punching through the 2" wooden backing - the only target I had on hand at the time. I tamped the SC after sealing it to
the target with a cornstarch/water thixotropic mix in a 5gal bucket which was also glued to the target. Ignition was electric, rear-center priming
with a CLD (A variant on Microteks design) and a 5g booster of cast PETN:ETN mix ala Rosco Bodine.
|
Hi Hex,
Just a side note about mixing weird and energetic HE stuffs...always think first of possible cross reactions....
1°)NH2-NH2.HN3 + NH2-NH2.HClO4 might free HN3 because NH2-NH2.HClO4 is very hygroscopic and acidic salt.
HN3 is bad news as is HCN (cyanide).
2°) PETN is not advisable in mildly acidic conditions...HP can then favourise hydrolysis and subsequent oxydoreduction, runnaway and explosion.
Nitric esters ought to be in neutral media for storage.
3°)Transesterification can be achieved between the alcohol nitrate and the perchloric amine salt.
R-O-NO2 + N2H4.HClO4 <==> R-O-ClO3 + N2H4.HNO3
So in the mix you get little by little some perchloric ester forming...
No need to remind you your last summer mishaps with glycerol triperchlorate and the sensitivity of the PE tetraperchlorate brother....
To my feeling, you should be very cautious with those shaped charges and detonators...they might explode spontaneous or upon the slightest induction
after a while.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
Hmmm. I never thought of that... However, I reckon if we keep the water out, it may be 'safe'. Perhaps I will experiment more - but keep the storage
tests FAR from people!
Another point - I am working on Hydrazine Nitroformate at the moment - trying to find a method to use. I am starting from Hydrazine Sulphate, and
could freebase to the Hydrazine Hydrate... Is this novel material worth my time? Oh, and can anyone tell me the formula of Ammonium Dinitramide - I
cannot remember it! My interest at the moment is Zero Carbon Energetics/low Carbon. I will explain why later on - I have this theory you see...
BTW, PHILOU, U2U me with your newest email address - the last one I tried got a wierd automatically generated reply in Dutch...
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by -=HeX=-  |
Another point - I am working on Hydrazine Nitroformate at the moment - trying to find a method to use. I am starting from Hydrazine Sulphate, and
could freebase to the Hydrazine Hydrate... Is this novel material worth my time? Oh, and can anyone tell me the formula of Ammonium Dinitramide - I
cannot remember it! My interest at the moment is Zero Carbon Energetics/low Carbon.
|
-HNF is not a novel material, it is for sure interesting. But slightly over oxygenated....
2 NH2-NH2.HC(NO2)3 --> 5 N2 + 5 H2O +2 CO2 + 3/2 O2
Ammonium dinitramide is simply NH3.HN(NO2)2
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
I was fairly sure it was 'relatively new'.
Still, it is interesting - I have a special love for the N2H4 based materials as they are just so interesting!
ADN is the next stop for my lab I think! It is maybe good for my rocket motors 
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Then you need effective cooling. I made KDN via the sulfamic acid route some years ago, and the CaCl2/crushed ice cooling that I used was barely
adequate even though it got to aroung -55 C.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ -=HeX=-
Each mol of the Hydrazine Nitrofrom salt yields 6.75 gas mols
4 { ( N2H4 )•HC(NO2)3 } => 4 CO2 + 10 H2O + 10 N2 + 3 O2
Trinitroethane is acid and should form a nitronate salt with hydazine
one mole of compound will yield 8 gas mols
2 { ( N2H4 )•CH3C(NO2)3 } => CO2 + 3 CO + 7 H2O + 5 N2
Trinitroethane has been known since late in the 19 th century. Originally made from
Methyl Iodide and Silver Nitroform in Acetonitrile. Organic Chemistry of Explosives- Agrawal , page 13
Cheaper reagents that should work as well would be Methyl Bromide and Lithium Nitroform in DMSO
∆Hf = - 26 kcal/mol (s), melting pt. 57 ºC, Boiling pt. 183 ºC , Density ρ = ( mol wt 165 / mol vol 1.0 ) = 1.65 gm/cc
http://www.chemspider.com/Chemical-Structure.11197.html
http://cdb.ics.uci.edu/cgibin/ChemicalDetailWeb.psp?chemical...
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=1549...
http://www.ncbi.nlm.nih.gov/sites/entrez?LinkName=pcsubstanc...
.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
@Franklyn,
To be of considerable acidity a nitrocompound must hold an hydrogen atom on the C holding the NO2 group (alfa position)...in CH3-C(NO2)3 there are
only H in beta position and thus the acidity will be very much lower than in nitroform.
Do you have pKa datas on 111-trinitroethan to check if an hydrazinium salt might form?
Maybe a mix of hydrazine and TNE might work even if the molecules aren't joined into a salt; but the density will be lower and it is possible both
doesn't mix well...
I would better suggest to get a better OB to work on the two following compounds:
1°) Cyanhydrazide nitroformate
N#C-NH-NH2 (cyanhydrazide) + HC(NO2)3 --> N#C-NH-NH3C(NO2)3
C2N6H4O6 --> 2 CO2 + 2 H2O + 3 N2
The introduction of a cyano group increases the energy output because it stocks some energy in the triple link.
2°) Hydrazinium dinitrocyanomethanate (dinitroacetonitrile salt)
NH2-NH2 + HC(NO2)2CN --> NH2-NH3C(NO2)2CN
C2N5H5O4 --> 2 CO + 5/2 N2 + 2 H2O + 1/2 H2
[Edited on 17-3-2010 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ PHILOU Zrealone
It does not seem that replacing the lone nitro group of Nitromethane with a
trinitromethyl group would make the methyl group behave much differently ,
if this is viewed as a primary methyl compound , ( ' methyl ' trinitromethane ).
Reduction of Trinitroethane to a dinitro compound is the observed reaction
with bases , adducts have not been documented , so may not form as such.
Your doubts are supported by the stated absense of H-bond doners in the
" Predicted Properties " subpart of the first reference I cited above _
http://www.chemspider.com/Chemical-Structure.11197.html
Organic Chemistry of Explosives - Agrawal , Quoted from page 54
" In Section 1.10.2.3 we observed that a base can react with 1,1,1-trinitromethyl compounds
to either remove an acidic proton or act as a nucleophile to displace a nitro group.
Section 1.10.2.3 , page 41 , ( imaged below )
An interesting azido derivative applying this path is desribed in the attached patent
1,1,1-Azidodinitroethane US patent 4472311
Organic Chemistry of Explosives - Agrawal , References cited on page 13
" synthesis and chemistry of 1,1,1-trinitromethyl compounds has been extensively reviewed "
9. F. G. Borgardt, P. Noble. Jr and W. L. Reed, Chem. Rev., 1964, 64, 19.
114. L. A. Kaplan, in The Chemistry of the Nitro and Nitroso Groups, Part 2, Organic Nitro Chemistry
Series, Ed. H. Feuer, Wiley-Interscience, New York., Chapter 5, 289–328 (1970).
115. (a) Nitro Paraffins (Ed. H. Feuer), Tetrahedron, 1963, 19, Suppl. 1; (b) Nitro Compounds (Ed.
T. Urbanski), Tetrahedron, 1964, 20, Suppl. 1.
.
Attachment: 1,1,1-Azidodinitroethane US4472311.pdf (191kB)
This file has been downloaded 1321 times 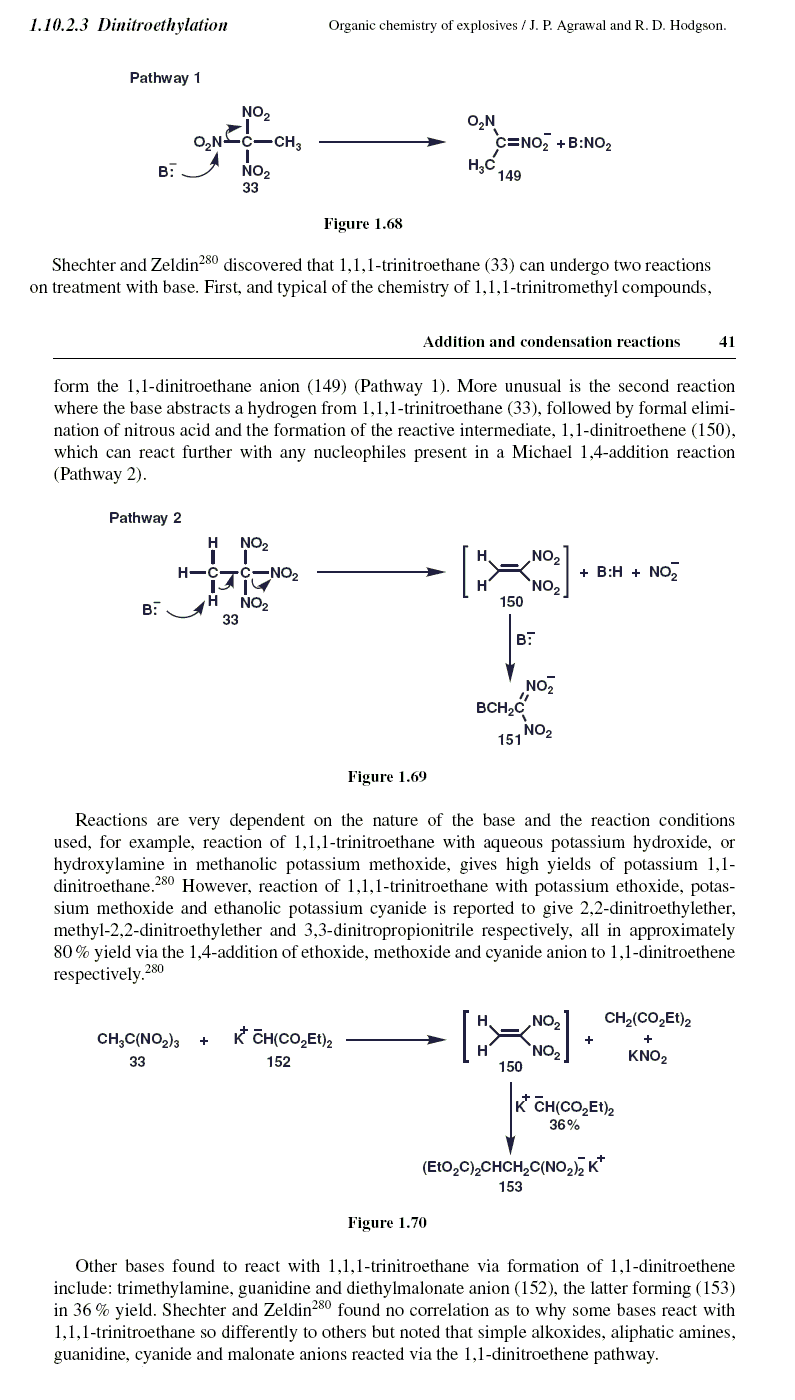
|
|
|
12332123
Harmless

Posts: 38
Registered: 14-11-2009
Member Is Offline
Mood: No Mood
|
|
Well, with regard to the need for alpha hydrogens, what about 1,1-2,2-tetranitroethane salts? Depending on how acidic it is it could either form
(NH3)2 C2(NO2)4 (with one extra oxygen atom per molecule), or 2 NH3-NH2 C2(NO2)4 (deficient by one oxygen atom per molecule), in both cases assuming
that the tetranitroethane is doubly deprotonated. Both of these should be somewhat more powerful than hydrazinium nitroformate given their better
oxygen balance.
|
|
|
12332123
Harmless

Posts: 38
Registered: 14-11-2009
Member Is Offline
Mood: No Mood
|
|
Or, if one doesn't mind hyperactive sensitivity, aminotetrazolium nitroformate could be synthesised. This has perfect oxygen balance and would likely
beat HMX by a fair margin (IIRC the dinitramide salt has a detonation velocity ~9500)
|
|
|
| Pages:
1
2
3
4
5
6
..
23 |