| Pages:
1
2
3
4
5
6
..
10 |
BromicAcid
International Hazard
    
Posts: 3270
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I don't think axehandle is going to be using a 100% oxygen feed, he is just going to be hooking a desiccant system to his air intake, look here
http://www.sciencemadness.org/talk/viewthread.php?tid=1691 I believe he might have mentioned that he would be using this for this reaction, if not
in this thread then in another relating to it.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Actually, BromicAcid, I intend to use pure oxygen, but fed very slooooowly. My experiment involving heating sulfur to the auto-ignition point showed
that the sulfur never catches fire in an air athmosphere, it only begins to boil from overheating.
The failure could have been due to temperature differences in the bottom where the heating element was located and the top, but I imagine pure oxygen
will work.
I don't intend to "whooosh" lots of pure O2 into the system, as that would probably lead to an explosion. I intend to feed it very
slowly, say 0.1-0.5 litres/minute.
Edit: I'm repeating the experiment now, but this time I use a very thin layer of sulfur in a cooking pot on the stove. I'd hate to have to
buy an O2 tank.
Edit2: No. The layer of molten sulfur (about 5mm deep) started to boil and sublime in the air. I let this continue for a few minutes before I gave up.
It never caught fire. THis is very strange. The boiling point is far higher than the auto ignition point, so why the &%¤# doesn't the sulfur
catch fire?
Edit3: I never anticipated the fscking burner to be the greatest obstacle. It's starting to lean towards the microwave solution after all.
Edit4: Either that, or finding an ignition wire capable of being heated to a red hot constantly, in an atmosphere of molten sulfur, air, and SO2.
Great. The only one coming to mind is Pt. That's out.
[Edited on 2004-3-19 by axehandle]
[Edited on 2004-3-20 by axehandle]
[Edited on 2004-3-20 by axehandle]
[Edited on 2004-3-20 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
I could not download your attachment.
Sulfur burns so easily in air! Why don't you put in the burner a tiny loop of thin NiCr wire heated white hot by a small transformer. Maybe even
touching the sulfur.
If you don't have the transformer, put it in series with a 100w light bulb. Or your heating coil.
Can't you leave a burning sulfur candle inside the vessel?
Can't you leave a small hole with a plug, so that you can open it, light the sulfur with a match and then plug it again?
An oxygen tube seems too much trouble.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
NiCr wire would be destroyed, I think.
How's a sulfur candle constructed?
/A
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by axehandle
NiCr wire would be destroyed, I think.
How's a sulfur candle constructed?
/A |
Is... eh.... a candle! Made of... well, sulfur.
Serious: I have never seen a sulfur candle. Google gave me 10 results, none of them with a picture. I believe if you melt some sulfur, wet a wick
(piece of string) in it and put the wick in the fused sulfur, it will work as a candle. The sulfur will be the parafin/stearin and the wick will be
the... wick.
other idea:
Break the glass of a light bulb, put the lamp, connected, inside the burner. When temperature is OK, light the bulb. Boom: sulfur on fire. Well,
curtains on fire, walls on fire, axehandle on fire... nah! No good.
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
ROFL You're real nice *cough* 
But wouldnt the tungsten just burn with a quick flash? Or... does that flash explode sulphur....
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Would this help?

|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Eeeehhh. Hmm. I think I'll try to cast a sulfur candle with a thick cotton whick.
Tacho, you may just have saved me about 2 weeks of work and about 150 Euro. I feel more than a bit stupid for not coming up with this idea myself....

Edit: But the casting will have to wait until Monday. My stock of candle whick went empty last week.... 
Or I could perhaps melt a few ordinary candles, recover the wicks and boil them. That should get rid of most of the stearin.
Worth a try. May even do it today, if the weather is favorable...
/A
[Edited on 2004-3-20 by axehandle]
[Edited on 2004-3-20 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
I cant see the pic 
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Saerenyde, the flash would ignite the sulfur, but maybe the gases from hot sulfur inside the burner would explode.
The picture displays a ignition coil and a circuit to provide sparks.
This is the first time I post pictures linked to my new website, does anybody else have trouble seeing it?
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Hmmm.. your site doesnt load :|
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
axehandle,
Take a look at this picture, maybe you will be interested. Maybe this is the solution to your sulfur ignition problem, if the candle does not work.
Sorry about the site & pictures, I'll have to find out what is going on.

|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
I think a sulfur candle should be not too hard to make. I *think* that since sulfur has about 3 times as high an MP as stearin, the whick will need to
be thick. (This sounds very funny in Swedish since "whick" has two meanings, catch my drift...). Maybe it could be made from a string of
rockwool, if it needs to be incombustible. I will find out on Monday, because then I'll go out and get some whick.
Oh dear, that word should really be substituted by "burn-sustaining substrate".....
About the picture: Thanks, but I already have one of those, made for a very different project (which failed). It involved a battery powered car
ignition coil 40kV spark generator. I also have one of these (it's an 8kg heavy resin block 9kV 60mA NST): 

My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
New design (again).
Since multiple attachments can't be made, and the BB Code and IMG-tag is off, I have to make this a new post. Please bear with me, moderators.
This is a revised design. In fact, the cast aluminum vessel in the new design doesn't have to be cast, One could simply take a piece of 50x2mm
aluminum pipe and cast the bottom piece out of gypsum or phosphate cement. The connectors could be made from 15mm Al roundbar, and screw-in pieces
could be made from that using only a drill press and some machine-screw-making tools. They could even be copper or glass pipe in cork plugs. The heat
production will be low.
Edit: Now I feel I'm finally getting somewhere! Thanks for the idea, Tacho!
[Edited on 2004-3-21 by axehandle]
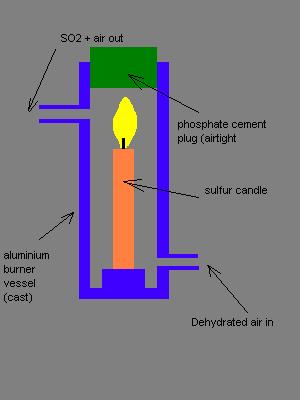
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Something is wrong here.
The thing that is wrong is that HTML, Smilies, BB Code, and img code are all available, but they're not showing up as available.
[Edited on 3-21-2004 by Polverone]
PGP Key and corresponding e-mail address
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Sulfur candle mold woes.
OK. This may make me stand out like an idiot, but I can't figure out how to make the mold for a sulfur candle. I've got lots of whick now,
and have been thinking a lot.
Paper is out, because it would stick to the sulfur (very fluent when molten).
Aluminum foil is out too (weight of sulfur would collapse the mold).
Anything else is out due to the high melting point of sulfur.
I need a material and a way to make identical, 200mm high, 20..30mm thick sulfur staffs with an integral whick (leading to the problem of sealing the
bottom of the mold while holding the whick in place).
I'm brainfried. Please assist...
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
First I would make a small prototype in a mold of aluminium foil, just to see if it works as we hope it does. You don't have to remove the foil
to test. Just 1cm long.
Paper would be perfect. Can't you paint it with sodium silicate (waterglass)? Maybe a thick sugar (syrup) solution? It would render the paper
"sulfurproof" after it's dry, but would easily dissolve in water, releasing the paper.
Another idea: use thick Al foil, the kind they use to make disposable dishes or reinforce it with paper. Cast the thing and put it in HCl or NaOH
solution. -> bubbles, fizz->no more Al foil.
Cast it sideways, in an easily removable mold in the shape of a groove. One side of your candle will be flat. So what?!
Cast it in an empty eggshell (to empty: make 4mm holes on each end and blow). Toss in acid and you have a nice looking egg shaped sulfur candle ready
to spoil christmas.
If latex resist molten sulfur, cast it in a condom and remove the latex in the usual way. (edit) Then think of an explanation about that yellow object
in your room.
[Edited on 24-3-2004 by Tacho]
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
*thinking about how I explained that clay sculpture I made to my parents* ("It's a self portrait! No way, it can't be that big.
etc" 
Good ideas. I especially like the syrup idea. It should render the paper mold impervious to sulfur, as well as make the cancle "washable" in
plain H2O afterwards. It's a pity I don't have any syrup at home right now, but tomorrow.... it's syrup time! The syrup idea also makes
it easy to make the bottom part of "candle" out of plaster of paris, thereby holding the bottom part of the whick in place. Thank you again,
Tacho!
Edit: No way that a condom would hold molten sulfur! Besides, I don't have the opportunity to test, my GF is on the pills and I don't have
any stockpile of condoms...
[Edited on 2004-3-24 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
BromicAcid
International Hazard
    
Posts: 3270
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
How about aluminum can, or sand molds?
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Yes BromicAcid, I've thought of that, but how do I keep the bottom part of the whick, as well as its entirety, in place?
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
BromicAcid
International Hazard
    
Posts: 3270
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Take an aluminum can, puch a tiny hole in the bottom and put the wick in, tie it in a knot so that it wont go back through, take a rod of some type
and lay it across the top of the can and wrap the wick around it till it is taught, then pour in the sulfur and when it dries unwrap the wick from the
rod and cut to desired size.
Cut the top and bottom off the can then cut down the side, roll it into itself till you get the diamater that you want then run a bunch of tape around
it or use a hose clamp to hold it in that shape. Put a rod at the bottom and wrap the wick around it do the same with a bar at the top to hold it
tightly. Press it into the dirt a few mm so that it forms a seal then pour in your sulfur. Viola, when it dries cut the tape and unroll the aluminum
and you've got your candle of whatever diameter you want.
[Edited on 3/24/2004 by BromicAcid]
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Too thick
Too thick candle, it needs to be 20-30mm in diameter. Good idea though, if beer cans came in 30mm diameter, which unfortunately, they don't.
I've actually dreamt about it. It would be the _perfect_ size, that was what my dream was about. The candle needs to fit in a 50x2mm aluminum
pipe with room to spare for the air feed. That's 46mm ID, 50mm OD. So 40mm would be good too, but I can't find anything here 40mm in ID...
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Get a big potato.
Drill a 2cm hole in it and cut in half or cut in half and carve a groove (a "V" shape would be easy to make with a knife and result in a
square candle) in each half.
Hold the two halfes(sp?) together with a rubber band, with the wick in place and cast sulfur candle. Let sulfur cool.
Separate the halfes, save the sulfur stick, put halfes in microwave, cook for 10
minutes. Put two big chunks of camembert cheese on top of potato. Put a leaf of parsley on each half, some black pepper, and plenty of salt. Serve
before camembert melts. Tell your friends it's "Batata au enxofre" by Tacho.
Edit: Oh! No, this won't work!. The melting point of sulfur is above the boiling point of water: you will end up with sulfur all over your face!
Do the camembert thing, though.
[Edited on 24-3-2004 by Tacho]
[Edited on 24-3-2004 by Tacho]
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Your McGyverism is only surpassed by your sense of humour, Tacho 
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Very honored axe, but I must apologize: I forgot to mention the best parting agent of all times: Soap goo!
This is serious: Go to your bathroom. If you are like most of us, you have a white soap bar (maybe light green or light pink). Lift the soap bar and
you will see that goo that is soap dissolved in a bit of water. Paint the paper with this goo and let it dry. The paper should end with a waxy look
and feel. Like... dry soap.
This is a strong parting agent. It's excellent for plaster of paris. It is the only efficient parting agent for epoxy resin. Well, there is
vaselin, but vaselin makes the... oooh.... that's off topic.
Try this before you try syrup. It's more available, smells better, and it's not sticky.
By the way, why did you choose syrup instead of table sugar in water?
The soap goo is probably good for aluminum. I think you didn't read Bromic Acid post carefully, he had a good idea.
|
|
|
| Pages:
1
2
3
4
5
6
..
10 |