| Pages:
1
2
3
4 |
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | I am thinking about using the same fine sand for capturing the Na |
Does it really exclude air well enough to be used in this capacity? Also, depending on what's in it (carbonate contamination e.g. CaCO3, oxides of
iron or copper or w/e) the sodium might react with it. And even if it's inert and doesn't let in air, you still end up with a fused brick of sodium
and sand that then needs to be melted... I think you are better off trying to get the oil to work; you could try bathing the exit pipes in live steam
or for that matter boiling water, which should transfer away heat faster than passive air cooling without risking solid sodium.
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Maybe molten candle wax (virtually pure paraffin) is an option as it does not react with Na.
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Other way to collect Na metal
Now I was thinking about another approach: attaching a closed metal can on top of the retort with the only opening being a narrow collar stuffed with
rockwool allowing the CO to escape (and flush the oxygen out of it) while the Na vapor condenses and freezes to the inner wall of the can. At the end
of the process the can can be taken off and the Na metal scraped off the walls.
Any comments ?
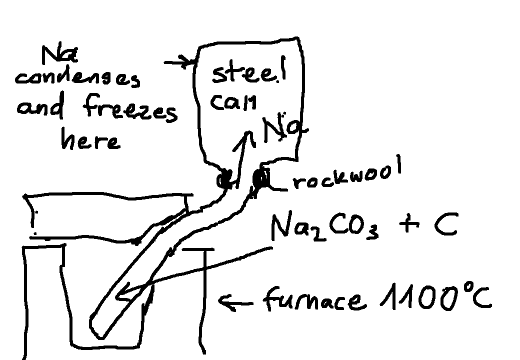
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
I tried an electric furnace
Here all my pics and test results of sodium making:
http://www.metallab.net/Na.php
Now I tried an electrical furnace (without actual reaction, just to test if it gets hot enough) see the page mentioned above under the heading
'electrical furnace'.
|
|
|
Picene
Harmless

Posts: 15
Registered: 5-10-2010
Member Is Offline
Mood: No Mood
|
|
What was your yield and purity like with this technique?
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
@Picene : No yield yet I am experimenting:
And here the so manyth episode:
I got the leaking issue under control now by putting the retort in a sand filled crucible to spread the heat evenly along the retort and to reduce the
oxidizing due to the sand layer. Furthermore I used a 20mm steel pile rather than 15mm. I can got no Stainless yet :-(
As the Kanthal furnace has to be rewired with thicker wire to get more power (700-1000W instead of 500W) I made a completely new small furnace heated
by a low flame propane (without extra air) and keep all openings closed (except the top exhaust hole) with refractory felt. The oil did not catch fire
anymore and the process ran smoothly (45 minutes) with a retort temperature (pyrometer peeked into the sandbox) of 1150oC.
But there was NO SODIUM at alll 
The inside of the retort tube was clean, and when I poured water into the cooled retort no hissing appeared (and did not leak from the bottom which is
a lttle good news).
During the process I saw it bubbling .
Where is the sodium gone ? In previous attempts I did get sodium.
Here a picture of the setup running:

|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
i wonder if encapsulating the retort in sand is insulating it? carbon as a reducing agent frustrated my first phosphorus attempts due to its extreme
temperature requirements...gladly though you have been able to control leakage in your system. it just seems to me in my humble, uneducated opinion
that instead of lowering your temp or making furnace conditions any calmer than you have been using that perhaps using rigid conduit (significantly
heavier walls) and the most fierce fire you can may lead you to the promise land. not sure if they have home depot where you live but an equivalent or
even electrical supply carry threaded nipples, caps, and 45-90 degree fittings that can be made airtight even if you have to weld them. at least in
that circumstance the thickness of the pipe will help you avoid burn through and give your retort the necessary strength to survive the heat needed to
start the reaction with carbon. this is the first retort design i used.
http://www.sciencemadness.org/talk/viewthread.php?tid=65&...
worked well with the aluminum reducing agent, but i haven't given it a try with carbon since the aluminum proved to work well. it was airtight and the
heavy construction may benefit your application.
[Edited on 11-10-2010 by Rogeryermaw]
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
@Rogeryermaw: I addressed the pipe bending issue as well by filling the one-side-squeezed tube with sand and plugging the other end with wood, so 45
op 90 degree nipples are not needed anymore and another leak risk is eliminated.
I have cleaned the oil capture cups.
Next test is a small test: three 15mm pipes
- Na2CO3 + C
- Na2CO3 + Al
- K2CO3 + C
with just small quantities (~ 1 gram) and one end (narrowed) open and heat them al three to 1100-1200oC and just to test which one is first doing to
issue Na or K vapors.
The one which works best I will repeat with capture under oil with the 20mm bended tube.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
i am hoping strongly for your success. i know we're all watching close! good luck!
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
I performed a test with three reactants !
Tonight I did a small test with three steel tubes with the following reagents as content:
Na2CO3 + Al
K2CO3 + C
Na2CO3 + C
Each tube was placed , one at a time, in a propane based furnace heated to 1200°C.
As is to be seen bright yellow flames appear from the tube which represent burning sodium metal flames. The potassium is not so clear but I saw
purplish flames appearing from the tube but I was just too late for reccording it with ther camera (second video). So this is a proof that in all
three cases sodium (or potassium vapor does appear.
So the reaction *DOES* take place !
<object width="480" height="385"><param name="movie"
value="http://www.youtube.com/v/hFh6Y8yr3ZM?fs=1&hl=en_GB"></param><param name="allowFullScreen"
value="true"></param><param name="allowscriptaccess" value="always"></param><embed
src="http://www.youtube.com/v/hFh6Y8yr3ZM?fs=1&hl=en_GB" type="application/x-shockwave-flash" allowscriptaccess="always"
allowfullscreen="true" width="480" height="385"></embed></object>
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
So I am still working on this but want to capture the Na metal in a different way, no oil but an empty container stopped off with glass wool.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
You are going to need to start using cylinders of argon to do that. Relying on the blanket from the reaction when the whole exit section is roasting
hot and there's vapour / molten sodium around, and jets of fire, is going to result in issues.
Even with a flow of argon, I'd be worried about it being blown out enough by a strong breeze that'd it'd start going.
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
@peach:
I want to keep the collector outside the furnace and out of reach of flames, etc. possibly insulated with rockwool.
Of course, Ar gas flushing is better but I don't have access to this.
The furnace is completely blanketed off so the flames escape only via the top hole as you see in the pix in earlier posts in this thread.
Earlier I tried a Kanthal furnace for the reason of no flames but that does not get hot enough.
I'll just try but this may not help.
[Edited on 2010-10-19 by metalresearcher]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I suspect you may be on your way to a very bad accident if you try running this without the oil and without an argon cylinder.
Think about what happens when you slice through solid, room temperature sodium. The stuff coming out your exhaust will be gaseous / molten and white
hot.
Any oxygen getting into the result will drive it ballistic. Perhaps quite literally.
If you can't afford or get a cylinder, you need to stick with the oil.
You may get lucky a few times, but that is not a reliable method of doing it - having the result dump out so hot and finely divided into a only
partially controlled atmosphere.
If the sodium ignites, it's going to be even worse than an oil fire.
They used to do chemistry like this in the beginnings, and produced materials like phosphorus and sodium using very basic setups. But they usually
sealed everything as a solid chamber, and they also had serious problems with the staff continually being set on fire or gassed to death. This was an
era where loosing a few of your kids to the cotton mill was considered normal.
[Edited on 19-10-2010 by peach]
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
The sodium and air make an explosive mixture you mean ?
Anyway wilh new attempts I'll stick to the oil (can I also use nonflammable vegetable oil ?) or go for electrolysis. I already made a setup but I am
scared for the hot lye.....
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
It's basically like magnesium ribbon, but it'll be even more prone to doing it. It goes white almost instantly when it's cut in the open atmosphere,
so it's oxidizing quickly without any extra heat as a solid.
Once it's burning, that can also burn inside a block of dry ice. Water will only make it worse.
I really think these are things you should already know if you're trying to make it. This is demonstrated to 16 year olds in chemistry, so it's fairly
basic stuff.
You're trying to produce an extremely reactive metal, which is not at all easy or safe, so you need to be more sure of what you can and can't use
yourself. It's not going to be as easy or safe as running thermite to get iron.
One member on a forum full of chemists has managed to produce a clean, reliable result thus far, and he's a real life physicist. Lots of people want
it, so that should be a warning of the difficultly involved.
Why do you want sodium so badly, do you actually need it for alloying, solvent drying or organometallics? If it's to have some for the sake of it, or
drop it water, you'd be better off buying it. If it's to solve the production method, you need to know about the oxidation, heat and fire problems and
be ready to buy things like argon to experiment.
It could be quite likely that your retort isn't producing much because it's oxidizing as it's coming out, that the atmosphere isn't inert enough.
Another reason for the argon, to test it actually works with that prior to relying on the self produced reducing atmosphere.
Try opening a lithium battery and watch how fast the metal oxidizes, the strips goes black within about 30 seconds. And sodium is further down the
table.
Lithium can be used for a number of the things sodium can, and does the same fizzing to nothing in water trick.
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Not for throwing it into the pond or other uses for that I can buy it you are riight.. It is just for making tiny amounts to make myself. I am
interested in metals and it is a real challenge for me to produce such a reactuve alkali met. And I know the dangers.
I have experience with metak melting (copper. silver. gold. cast iron) making furnaces. etc.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Right, but sodium is quite far away from any of those with regards to the fire problems. Gold is fairly difficult to react with anything bar aqua
regia. Whereas sodium will quickly react with the air if you plop a lump of it out on the desk.
The same is true at the other end of the table. The noble gases, like argon, are next to impossible to react with anything. But the halogens and
reactive acid / base gases will eat through the cylinders, and they're in the next column on the table and in very similar looking cylinders.
I'm 100% behind the effort you're putting in and not trying to call you dim, I'm just double checking you know why I might think it's a bad idea to
try that at those temperatures with a setup that could end up contaminated with oxygen or moisture. It will be so easy for it to go up in flames,
sputter at you or explode in a way that will be next to unstoppable. Len had problems with that even when he was running it at far lower temperatures
and with a fairly high tech bit of gear - popping and sputtering.
Even the guys with degrees in chemistry at Cambridge have had problems with sodium stills setting people on fire. So much so that they'll spend tens
of thousands on dedicated rooms for them now.
The guys who weld things use argon cylinders all the time, and they're only dealing with molten steel.
I'd suggest you get some if only to test the retort is functioning - for example, that you're not seeing poor results purely due to oxygen lurking
around.
BOC will now rent cylinders on a monthly basis. Grab one of those suckers, try the oil again but blow the retort full of argon before warming it up,
then trickle it in as it runs. If you have a neighbour who welds, see if they'll let you borrow their cylinder if you fill it back up. Some of the
welding shops might let you use theirs as well given that you're trying to make a metal, provided you fill it back up. A cylinder refill is cheaper
than the rental.
It really may be that simple. That you run the argon in and get some sodium out from the same design. Then you'll know... "ahhh.... the atmosphere is
off" or "right, it's the temperature / mix".
There aren't too many variables at work here, so the atmosphere is a strong contender for the error given the metal and process involved.
I've sat and watched organic reactions at room temperature fail due to poor atmospheres - gradually turning brown.
[Edited on 19-10-2010 by peach]
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
@peach: thanks.
I found some online shops who sell discardable Ar cylinders and pressure regulators for less than $100.
That may be a serious option not only for Na making but also for other experiments which don't like oxygen.
I think even melting copper can be done under Ar as liquid copper absorbs oxygen and makes it brittle which results in cracks when I am rolling it.
[Edited on 2010-10-20 by metalresearcher]
|
|
|
Polverone
Now celebrating 21 years of madness
|
Thread Split
21-10-2010 at 05:51 |
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
First attempt to electrolyze NaOH
Finally I attempted to electrolyze NaOH . I saw sodium forming but could not collect it yet.
Here my results..
Does anybody have suggestions ?
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
http://www.youtube.com/watch?v=faorfmRkCv0&feature=chann...
If this has been posted before I'm sorry, but if not I'd have to say this looks like the easiest way to go about it. Probably not pure but hell, it
could be a jump off point. No need for big furnaces or electrolysis. Just a pot you don't want, some NaOH, Mg, a fuse, and some mineral oil.
The hole can thing doesn't seem like too great of an idea but hey what the hell.
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
I already saw this vid, and tried it with Mg filings and NaOH which I put into a 2.5cm diameter and 5cm long steel tube closed and welded at one end
and heated it. Just before red hot it reacted but the problem is (as you can see in this vid) how to isolate it ? With electrolysis or a Na2CO3 + C
retort furnace it is easier I think.
|
|
|
| Pages:
1
2
3
4 |