| Pages:
1
..
35
36
37
38
39
..
44 |
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  |
I was expecting that Cu2+ would oxidize SO2.
Fe(OAc)3 may be a somewhat stronger oxidizer, but it is much harder to dry. AgOAc is easy to dry, but expensive and probably insoluble. Pb(OAc)4 is
cheaper but toxic. Perhaps Fe2(SO4)3 could be used? |
I didnt find any reference for making Ac2O by this method
(i think this is time that you test your idea and publish it)
[Edited on 23-11-2018 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
j_sum1
|
Thread Closed
30-11-2018 at 14:57 |
j_sum1
|
Thread Opened
1-12-2018 at 16:26 |
NZniceguy
Harmless

Posts: 48
Registered: 16-9-2017
Member Is Offline
Mood: No Mood
|
|
Has anyone actually tried the Sodium Pyrosulphate and Sodium Acetate method from the Henry Dreyfus patent? I have seen that some people have had
success with it on another forum but it looked like it was quickly dismissed in this thread without anyone actually trying it.
It is also supposed to work with Propionate salts to get Prop. Anhydride.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
IIRC tests by SM members showed that the method does work for propionic anhydride but does not work for acetic anhydride.
One possibility is to use pyrosulfate to make benzoic anhydride and then react this with anhydrous metal acetate to get acetic anhydride. Benzoic
anhydride is probably a good case for this rxn since it is not enolizable or easily oxidized. Also sodium benzoate can bee had in bulk and IIRC is not
hygroscopic.
[Edited on 12-7-2019 by clearly_not_atara]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  | IIRC tests by SM members showed that the method does work for propionic anhydride but does not work for acetic anhydride.
One possibility is to use pyrosulfate to make benzoic anhydride and then react this with anhydrous metal acetate to get acetic anhydride. Benzoic
anhydride is probably a good case for this rxn since it is not enolizable or easily oxidized. Also sodium benzoate can bee had in bulk and IIRC is not
hygroscopic.
[Edited on 12-7-2019 by clearly_not_atara] |
I tried reaction of Pyrosulfate with acetate and propionate salt without any success.I will be so happy if someone show this method is not bullshit
and Henry Dreyfus is not lier !
Ac2O from Benzoic anhydride is possible and i mentioned this method on page 33
Chemistry = Chem + is + Try
|
|
|
NZniceguy
Harmless

Posts: 48
Registered: 16-9-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Waffles SS  | Quote: Originally posted by clearly_not_atara  | IIRC tests by SM members showed that the method does work for propionic anhydride but does not work for acetic anhydride.
One possibility is to use pyrosulfate to make benzoic anhydride and then react this with anhydrous metal acetate to get acetic anhydride. Benzoic
anhydride is probably a good case for this rxn since it is not enolizable or easily oxidized. Also sodium benzoate can bee had in bulk and IIRC is not
hygroscopic.
[Edited on 12-7-2019 by clearly_not_atara] |
I tried reaction of Pyrosulfate with acetate and propionate salt without any success.I will be so happy if someone show this method is not bullshit
and Henry Dreyfus is not lier !
Ac2O from Benzoic anhydride is possible and i mentioned this method on page 33
|
I have seen reports that the Propionic salt and Pyrosulphate has worked for people but I just tried it and it failed. Im unsure if I managed to get
Pyrosulphate from Bisulphate though which may have been the problem.
What happened when you tried the propionic salt with pyrosulphate?
Has anyone here had success with the pyrosulphate method?
[Edited on 5-8-2019 by NZniceguy]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
I just got something like propionic acid each time(I even used dry Merck pyrosulfate and propionic salt)
My advise : dont waste your time by this method
Chemistry = Chem + is + Try
|
|
|
nleslie321
Hazard to Self
 
Posts: 62
Registered: 7-11-2017
Location: krakatoa
Member Is Offline
Mood: just right
|
|
Making acetic anhydride is very easy and straightforward if done safely using ketene. The key word here is SAFELY as ketene is on par with hydrogen
cyanide. Just follow the setup that doug uses on dougs lab youtube channel or a variation of the setup. Ive had no problem using this method and if
done safely it is extremely simple to achieve in a amature home lab with a fume hood. Ive also used the S2Cl2 and sodium acetate method which is also
quite easy but a lot more work and messy/smelly because you have to synthesize the S2Cl2. I don't know why so many people ask about making acetic
anhydride when its so simple to synthesize. I can also vouch that the pyrosulfate method is bullshit for acetic and for propionic anhydride. I had no
luck with either i did however find propionic anhydride extremely simple to synth via ketene and propionic acid.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Ok, I had another thought.
Suppose you gas HCl to a solution of succinic anhydride and GAA. The result should be an equilibrium between succinic monochloride and acetyl chloride
(I think this leans left, but whatever):
AcOH + HOOCCH2CH2COCl <> AcCl + HOOCCH2CH2COOH
If this solution is heated, acetyl chloride should boil away first by Le Chatelier’s principle. It is dramatically more volatile than anything else
in the flask.
This is particularly attractive because succinic anhydride can bee had by simply heating succinic acid to 200-250 C. See:
https://patents.google.com/patent/US3957830A/en
EDIT: this might also work for benzoyl chloride or [m]ethanesulfonyl chloride!
[Edited on 15-12-2019 by clearly_not_atara]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
2 Ideas:
Ozonolysis of acetylene
"Decomposition" of acetate salt
I know Wikipedia isn't necessarily a great source, but given the accessibility of both ozone and acetylene I think it's worth some thought. Given the
fact that it would be a gaseous mixture of an explosive gas and an extremely strong oxidizer, I'm not recommending that anyone without the proper
equipment and experience try.
Again from Wikipedia, heating anhydrous Zinc Acetate apparently releases Acetic anhydride in a vacuum, leaving the basic Acetate salt. A good enough
vacuum might be hard to pull, but again due to the accessibility of acetate salts it may be worth a shot.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Ozone + acetylene sounds like a really terrible idea.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
It sounds like one of the worst ideas I have seen around here. If you want an explosion I would recommend TATP before I would recommend this one.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Quote: Originally posted by Tsjerk  |
It sounds like one of the worst ideas I have seen around here. If you want an explosion I would recommend TATP before I would recommend this one.
|
Great minds think alike! So alike that I explicitly said it should not be attempted by anyone who's not equipped to deal with it. I can't tell if you
actually think I want an explosion, but I'd rather sit on a nail than make TATP again. Do you have anything thoughts on the Acetate salt?
Given the fact that it would be a gaseous mixture of an explosive gas and an extremely strong oxidizer, I'm not recommending that anyone
without the proper equipment and experience try.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Wasn't this combo covered in Igntition? It wouldn't be above average for those nutjobs. But yeah, that sounds like premeditated *kaboom*.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by njl  | 2 Ideas:
Ozonolysis of acetylene
"Decomposition" of acetate salt
I know Wikipedia isn't necessarily a great source, but given the accessibility of both ozone and acetylene I think it's worth some thought. Given the
fact that it would be a gaseous mixture of an explosive gas and an extremely strong oxidizer, I'm not recommending that anyone without the proper
equipment and experience try.
Again from Wikipedia, heating anhydrous Zinc Acetate apparently releases Acetic anhydride in a vacuum, leaving the basic Acetate salt. A good enough
vacuum might be hard to pull, but again due to the accessibility of acetate salts it may be worth a shot. |
Decomposition of zinc acetate has been brought up again and again, but nobody here seems to have actually heated zinc acetate at 2 mm pressure and 250
degrees C to try it out for themselves.
These the parameters quoted in old sources for this decomposition reaction.
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Quote: Originally posted by SWIM  | Quote: Originally posted by njl  | 2 Ideas:
Ozonolysis of acetylene
"Decomposition" of acetate salt
I know Wikipedia isn't necessarily a great source, but given the accessibility of both ozone and acetylene I think it's worth some thought. Given the
fact that it would be a gaseous mixture of an explosive gas and an extremely strong oxidizer, I'm not recommending that anyone without the proper
equipment and experience try.
Again from Wikipedia, heating anhydrous Zinc Acetate apparently releases Acetic anhydride in a vacuum, leaving the basic Acetate salt. A good enough
vacuum might be hard to pull, but again due to the accessibility of acetate salts it may be worth a shot. |
Decomposition of zinc acetate has been brought up again and again, but nobody here seems to have actually heated zinc acetate at 2 mm pressure and 250
degrees C to try it out for themselves.
These the parameters quoted in old sources for this decomposition reaction. |
If i have nothing going on, I might try it this weekend just for the fun of it. People like to suggest things, not read the thread and then not try
it.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
G-Coupled
Hazard to Others
  
Posts: 287
Registered: 9-3-2017
Member Is Offline
Mood: Slightly triturated
|
|
Quote: Originally posted by Abromination  |
If i have nothing going on, I might try it this weekend just for the fun of it. People like to suggest things, not read the thread and then not try
it. |
I feel that would be a most valuable contribution to the sum of knowledge on this fine forum. I eagerly await your results, should you perform this
experiment. 
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Quote: Originally posted by G-Coupled  | Quote: Originally posted by Abromination  |
If i have nothing going on, I might try it this weekend just for the fun of it. People like to suggest things, not read the thread and then not try
it. |
I feel that would be a most valuable contribution to the sum of knowledge on this fine forum. I eagerly await your results, should you perform this
experiment.  |
I will soon, but weekend was spent fumehood building. Ill read through the whole thread and do some more research first. I dont think it will work,
but why not try
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Quote: Originally posted by byko3y  | I want to put an end to the discussion of distilling acetate for preparation for acetic anhydride.
https://www.sciencemadness.org/whisper/viewthread.php?tid=9&... - link to the original article on Zinc Acetate.
https://www.sciencemadness.org/whisper/viewthread.php?tid=9&... - report about results by very same Waffles SS.
Now let's look inside the article - at the mass spectrum graph they recorded:
Here I made a spectrum for 260°C :
Now let's take a look at the reference mass spectrum of acetic anhydride from NIST:
Do you see the similarity? Because I don't. How the hell could those douchebags find acetic anhydride in their spectrum - god only knows. Some more
spectrums of possible reaction products for reference (major ions in orded of decreasing intensity):
http://webbook.nist.gov/cgi/cbook.cgi?ID=C108247&Mask=20... - Acetic anhydride: 43 (faint 42)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C64197&Mask=200... - Acetic acid: 43, 45, 60 (15, 42)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C67641&Mask=200... - Acetone: 43, small 58 (42, 15)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C123422&Mask=20... - Diacetone alcohol: 43, small 58, 59 (101)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C141797&Mask=20... - Mesityl oxide: 83, 55, 98, 43, small 29, 39 (a lot of faints)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C630080&Mask=20... - Carbon monooxide: 28
http://webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Mask=20... - Carbon dioxide: 44 (28, 16, 12)
http://webbook.nist.gov/cgi/cbook.cgi?ID=C7732185&Mask=2... - Water: 18, small 17
http://webbook.nist.gov/cgi/cbook.cgi?ID=C74828&Mask=200... - Methane: 16, 15, small 14, 13
http://webbook.nist.gov/cgi/cbook.cgi?ID=C74840&Mask=200... - Ethane: 28, small 27, 30, 26, 29
http://webbook.nist.gov/cgi/cbook.cgi?ID=C75070&Mask=200... - Acetaldehyde: 29, 44, 43, 15, small 42, 14
(there're similar spectrums on restek website, e.g.
http://www.restek.com/compound/view/67-64-1/Acetone - Acetone
http://www.restek.com/compound/view/123-42-2/Diacetone%20alc... - Diacetone alcohol )
Now let's try to analize the spectrum of gaseous products at 260°C: 15, 43, 16, 44, 45.
15 m/z is a methyl ion CH3+.
16 m/z is not oxygen but methane ion CH4+. Prevalence of it together with 15 m/z means methane is a major product. The fact that intensity at 15 is
higher than 16 means there are other methyl-producing substances.
44 m/z is a carbon dioxide ion CO2+. CO2 is another major product of the reaction.
There's no 18 ion meaning no water detected. That could be explained by the fact decarboxylative ketonization does not generate water but carbon
dioxide is produced instead.
45 m/z is an unknown ion. It could be acetaldehyde, but this substance has a very distinctive 29 ion which was not detected by authors. Acetic acid
has smaller ion 45, but it also has a smaller definite 60 ion, which was not detected too. My guess: 60 m/z ion was ignored for some reason (small
relative intensity?) and acetic acid is formed in substantial amounts.
43 m/z is acetyl CH3CO+. Could be derrived from any molecule with acetyl fragment. Probably that's acetone and diacetone alcohol which both have much
smaller other ions.
I'd guess the detected products were methane, CO2, acetone, diacetone alcohol, small amount of acetic acid. There's also a lot of solid products,
formed by polymerization of acetone, but those were not volatile enough to be detected.
Update: corrected ion number for acetaldehyde. Now acetic acid is the only option for 45 m/z.
[Edited on 28-2-2018 by byko3y] |
Dont try unsuccessful methods !.
[Edited on 7-2-2020 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Waffles is right, I’m still not sure why we get so many people wanting to try stuff we know doesn’t work.
But nobody wants to work on the methods that do or might work. S2Cl2 is too hard, let’s build a ketene lamp. I don’t get it. Because ketene is
safer than chlorine, somebody thinks.
I admit I’m just mad because I posted a kind of unlikely idea and everyone ignored me and then went for these obviously terrible ideas. Obviously
I’m wasting my time
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Hope I don't sound hostile, but I read through the entire thread and determined the same. Someone mentioned an idea, and the same idea had been
mentioned several times by others who had not read the full thread. I looked into it, found the idea to be futile and did not bother with saying so
assuming anyone else who wanted to would do the same. It was simply an accessible idea, and even though I have no use for acetic anhydride I still
found it worth looking into.
If you have an idea, test it yourself and report back. No one is stopping you.
[Edited on 2-8-20 by Abromination]
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Tamerlane
Harmless

Posts: 5
Registered: 12-11-2019
Member Is Offline
|
|
I had an idea for forming acetic anhydride by transacylation with a more easily prepared cyclic anhydride. For example, maleic anhydride is freely
soluble in aromatic solvents, whereas maleic acid is almost completely insoluble, on the order of tens of milligrams per liter of toluene. If there is
any sort of equilibrium formation between maleic and acetic acids and anhydrides in toluene, the disproportionation would be driven forward by the
extreme insolubility of maleic acid in the solvent.
To my dismay, according to Solvolytic Reactions of Cyclic Anhydrides in Anhydrous Acetic Acid (J. Pharm. Sci.), there is no appreciable
equilibrium reaction between maleic or phthalic anhydrides and acetic acid. However, the equilibrium reactions between saturated cyclic anhydrides
such as succinic anhydride, glutaric anhydride, and trans-1,2-cyclohexanedicarboxylic anhydride with acetic acid DO take place. But the solubility
characteristics of those anhydrides in aromatic solvents are much less favorable! 
From what little information I've found, succinic anhydride doesn't seem to be too soluble in aromatics, and the free acid is possible somewhat
soluble as well. Glutaric anhydride/acid could perhaps be more favorable but I haven't found much in the way of solubility data. Perhaps if
appropriate solvents could be found, the reaction could be made to work. Or perhaps in the case of maleic anhydride, a sufficiently long period of
time could coax the sluggish equilibrium forward. It really seemed like I was on to something!
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Tamerlane: I had considered the same with Le Chatelier's principle applied through volatility:
RR=(C2O3) + HCl <> HO2CRRCOCl
HO2CRRCOCl + AcOH <> AcCl + HO2CRRCOCl
AcCl is much more volatile than any of the other rxn components and should distill out. In this case GAA is the ideal solvent.
However, while the preparation of succinic anhydride by thermolysis of succinic acid is commonly talked about, it is difficult to obtain a pure
product in decent yield. I wonder if sodium pyrosulfate or metaphosphoric acid could be used to make succinic anhydride?
[Edited on 3-5-2020 by clearly_not_atara]
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
On the ketene lamp concept, could ketene be formed by just distilling acetone trough SS tube that is drawn through ceramic wool furnace heated with
propane burner?
It could be more feasible to produce this than operating with nichrome wire. Put acetone vapor through bottom inlet, heat the inside of furnace to
800C and out comes ketene, which is cooled with reflux condenser with receiver for any side reactants and the final gas ketene led into GAA.
This type should be easily scalable due to tube length being the factor of pyrolysis capacity. The temp of 800C would be very easy to maintain with
ordinary high pressure propane torch.
The joint between steel and glass between the pyrolysis tube end and the condenser would be an issue though. The gas would be very hot leaving the
furnace and it would quickly melt any plastic joint. Making a larger tube ending and turning a graphite adapter would be an option.
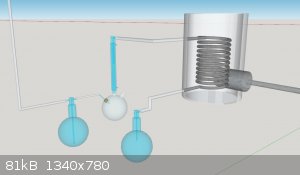
[Edited on 14-6-2020 by Refinery]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
As you know ketene(Ethenone) is very toxic and working with this compound is really dangerous.
I wonder why someone here didnt try any other possible method?
I didnt see someone to try reaction of DCC(N,N'-Dicyclohexylcarbodiimide) with acetic acid ? (I tried it before and this is very easy to get Ac2O from
DCC)
Or why someone didnt try NaPO3(Sodium Metaphosphate) with AcOH/AcONa according to GB299342 ?
Or Cleavage of Acetoxysilanes to Ac2O ? (I tried it before and that is very easy and high yield)
MECHANISM OF REVERSIBLE CLEAVAGE OF ACETOXYSILANES TO SILOXANES AND ACETANHYDRIDEJ.POLA, M.JAKOUBKOVA and V.
CHVALOVSKYInstitute of Chemical Process Fundamentals,Czechoslovak Academy of Sciences, 165 02 Prague- Suchdol
Or Isopropenyl acetate or Vinyl acetate method (i tried those too)?Or...
Reactions of Isopropenyl AcetateHUGH J. HAGEMEYER, JR., AND DAVID C. HULLTennessee Eastman Corporation,
Kingsport, Tenn.
Why dont try or research easier and safer methods?Why?
[Edited on 17-6-2020 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
monolithic
Hazard to Others
  
Posts: 436
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Where can I find a copy of this patent? Google is not showing anything.
|
|
|
| Pages:
1
..
35
36
37
38
39
..
44 |