| Pages:
1
..
33
34
35
36
37
..
48 |
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Definitely an electrical issue. The computer power supply is probably going over the Amps that the 12V output is capable of supplying.
They can be fickle to get to come on and off too if you have no load on the 5V supply. Put a small bulb on the five Volts supply side. Use a switch
between the green and black wire to turn supply on and off as the supply will 'behave' better using this method.
Look up the solubility of KCl on Wiki. Saturated solutions are OK. Supersaturated solution are pointless as you will get KCl coming out of solution as
a small amount of time passes unless you heat some more.
Dann2
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
@ Dann2
Thank you.
I realize that salt will fall out so I wait approx 2 hours (perhaps that really is not enough?) but have come to think that I might be getting
chlorate at the 8 hour mark, if the current is high enough. In a 2 liter cell 15A is enough to make that thing almost boil. I tied an experiment along
those lines last night and am waiting to see what will happen after a week. I'm recording every detail so that I can simply measure out what will be
the highest level of KCl the bring can handle.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
2 liters is a pretty small cell, so I am not surprised you are overheating it with 15 amps. You can lower the current, create some form of external
cooling, or change out into a larger cell.
Immersion of the 2 liter cell into a large pan of water, and directing a fan over the surface of the water, is a crude but surprisingly effective way
to drop the cell temp probably 20 to 30 degrees. But I think your best answer is to make a larger cell.
My technique lately for getting good saturation is to mix my liquor at 60 degrees, but add only enough salt that would dissolve at 50. After the salt
dissolves, get it going quickly, and it will never get below 55 degrees, and there will be no chloride precipitate. But this method only works if you
know SPECIFICALLY what temperature you maintain at X working amps. I've known for a long time that my setup will run at 60 degrees at my standard 50
amps.
This sort of maneuver can easily double the yield over a room temp KCl solution.
Lambda - TY for the kind words! But I am simply following the lead of many who have been here before.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
There is some KCl solubility info at this address:
http://www.oxidizing.110mb.com/chlorate/potassiu.html
I have been running my Na Chlorate cell (2.2 litres) with different sized Cathodes to find out if that makes a difference to CE. I am particularly
interested in very large Cathodes (low CD on Cathode) with no additive to see if they drastically reduce CE.
The results are a bit of a mess. The times I am allocating to the different Cathodes is far too short to see a trend. It's all rather confusing. I
will have to run the whole thing again using larger (6 days or so) times for different Cats.
Anode is Graphite, approx. 34mA per cm squared, pH is controlled at around 7 (with 0.6cc 12% HCl per hour), four Amps steady into cell (CC supply)
%CE was like this:
Start to day two CE = 72% (small and large steel Cat's used during this time as I was 'weathering' the large steel Cathode and not really trying to
monitor anything and also waiting for cell to come to steady state as far as acid addition is concerned)
Day three (small Ti cat) 43% CE
Day four (large Ti cat) 0% CE
Day five and six (small Ti cat) 0% CE !!!!!
Day seven (large steel cat) 95% CE
Day eight and nine (still large steel) 46% CE
Day 10, 11, 12, 13, 14 (small Ti cat) 48% CE
Chloride concentration now at 192 grams per litre.
Day 15 to 20, MMO Anode with small Ti cats inserted giving 73% CE (and cell still going). I increased the Current to 8 Amps too when MMO inserted
BTW.
The cell is possessed!
I will check some of the more daft looking figures/titrations.
The only useful figure I will get out of this cell run is:
What concentration of Chloride an MMO Anode will take Chloride to when let run and run?
Dann2
[Edited on 15-2-2010 by dann2]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
@ Swede:
Yes, 15 was complete over-kill. But I was so curious to see how the PSU would handle it (fed it through a 75W resistor because the PS is a 30!) I
watched as gas fairly flowed out of the vent hose! But since it did not appear to get over 80C I gave it a few hours, it seemed to calm down a bit.
The technique you described is helpful. That's something I have realized was appropriate: to get the solution started at 50-60 and -with haste; get it
in a 5gal. Bucket Cell. One serious problem is not being able to see well into a large non-transparent cell while it's running.
@Dann2:
You've taken it ( a 2L cell) to 20 days? The information is very interesting. I've also wondered if there is a point of diminishing returns: just as
I've often thought of what would be optimum current for what volume and saturation point. I haven't been doing this long enough to even formulate some
of the idiosyncratic elements, but a lot of the questions have been touched upon. Thanks for the solubility link. This is pretty interesting stuff.
[Edited on 16-2-2010 by quicksilver]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
@Quicksilver
Don't pay much heed to the efficiency figures above as they are not really of any use.
If you are getting 54% current efficiency (cell not pH controlled) you will get 1 gram of Sodium Chlorate per Amp every 167.76 minutes, or if you
like, 0.00596 grams Chlorate formed per minute per amp which is the same as saying that 0.00327 grams Sodium Chloride is used up per minute per Amp.
Got back to testing Lead Dioxide plating solution for Nitrites. The aquarium test kit works OK. The blue colour of Copper Nitrate (if you have Cu
Nitrate in solution) does not interfere as it is too diluted and not a strong enough blue.
It turns out my old plating solution (sitting for years AFIAK) has (evil) Nitrites in it as can be seen from picture. I added Nitrites to some of the
old plating solution and got positive tests but then decided to do a 'blank' (no actual Nitrites added) and still got a positive result.
I had to make up a small amount of new plating solution with Lead Nitrate and Cu Nitrate. I added approx. 0.003 grams Nitrite to 1.5cc of plating
solution (0.2%, figures very rough BTW) and got a clear immediate positive. The new plating solution on its own (no Nitrites added) gives a negative
for Nitrites (RHS of picture).
The drops of test solution stay at the top of the Plating solution unlike when they are put into pure water where they sink to the bottom.
Made some Bismuth Nitrate. Added 70% Nitric acid to Bismuth and heated a bit to hurry up reacting the larger lumps and then evaporated away the
Nitric acid.
Dann2
[Edited on 17-2-2010 by dann2]

|
|
|
ninefingers
Harmless

Posts: 13
Registered: 16-2-2010
Location: Cactus Country Tucson AZ
Member Is Offline
Mood: Vulcan don't have moods
|
|
This is probably old hat to you guys, but:
I worked on switching power supplies for years; most need at least 1/3 their rated output just to start (that can be removed when the cell is
connected.)
Computer p/s need a "power good" wire connected to ground to start. This is usually green--look up the mfgr/model on the web and see. They need the
main +5v loaded to about 30% start, also. (I've had poor luck with these).
No matter what P/S you use, use heavy wires and distance it from the cell unless it has a Good lid (The salty/chlorate spray is Very corrosive)
Ti anodes seem to corrode right at where they touch the air. That DMV can be painted and they will then work well. Even the copper rods I use to
hold my anode and cathode get corroded, so I paint everything above the water level except exactly where I need electrical contact. That gets graphite
grease.
My graphite electrodes I got from a salvage yard. They are cut to about 1" square by 12"long; I use two of them. I soak 'em 3 days in Linseed oil (I
have a gallon of it). At all hardware/paint/home stores. I think old motor oil or vegetable oil would work too, but messier.
Lead Peroxide has an extremely high resistance unless you use an old car battery grid. I haven't tried an old battery yet; I was going to run about
30 volts to it and have a trough of electrolyte above the cell vents, with plastic tubes sealed to it and the cells. Just pasting PbO2 to a plastic
tube or lead doesn't seem to work well--it takes 60 volts to push 1 amps thru it; it gets Hot. A plastic tube Melts. I was using a 100W light bulb
as a current limit; it glowed Brightly. I used lab grade PbO2; it still Sucks.
My old battery is a bit sulfated; be careful of that. Charge an old battery a good week; then dump the electrolyte (or use for H2SO4) and replace
with distilled water. Always keep cells Wet with distilled Water Only. If exposed to air, the cells may get badly oxidized and not ever work. I
have a de-sulfator circuit I need to finish and try on this old battery before I get the plastic trough/tubes sealed so they Stop Leaking--this is a
Pain. When this is ready, of course, then the water may be dumped and the salt
water or whatever you are electrolysing added. When this is ready, of course, then the water may be dumped and the salt
water or whatever you are electrolysing added.
Just plain Lead works pretty well. I also use it in electroplating.
I don't see why Gold won't work. I am trying to get some gold- filled chains to try. (Gold-filled is much thicker than plated; it is forced over the
base metal.)
I tried a so-called Pt "wire" I got on eBay; it was a rip off. That guy got a
lot of complaints--be careful buying precious metals now. A Pt/Ir plated Electrode was the same price ($55). A friend uses a Pt anode AND Cathode to
make chlorates. It is Very Clean; can take enough current to make perchlorate directly and burn all the NaCl/KCl out. What is left is clean and Pure.
No freezing and filtering to purify. That guy got a
lot of complaints--be careful buying precious metals now. A Pt/Ir plated Electrode was the same price ($55). A friend uses a Pt anode AND Cathode to
make chlorates. It is Very Clean; can take enough current to make perchlorate directly and burn all the NaCl/KCl out. What is left is clean and Pure.
No freezing and filtering to purify.
(We now together have about 20 Pounds of chlorates/perchlorates of varying quality. We can't use it all in 5 years. He is now attempting
hydrogen fuel for his car with his cell. Making chlorates electrolytically is messier and a much longer process than boiling Bleach; but the raw
materials (salt) aren't as suspicious or expensive as, say, 10 gallons of bleach. Perhaps tablets for a pool is a better idea, I haven't tried it.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ninefingers  | This is probably old hat to you guys, but:
Ti anodes seem to corrode right at where they touch the air. That DMV can be painted and they will then work well. Even the copper rods I use to
hold my anode and cathode get corroded, so I paint everything above the water level except exactly where I need electrical contact. That gets graphite
grease. |
Hello Ninefingers, (one assumes you were born with the extra one and still have your two thumbs?  ) )
Can you explain what your "Ti Anodes" consist off?
I will be giving the Lead and Gold Anodes a miss. They have been suggested 1001 times.
Cheers,
Dann2
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Perchlorate from LaserReds MMO!!
Hello,
I have been running an MMO Chlorate cell for some time.
The MMO used here was 'LaserReds' MMO as sold on EBAY. Exact type of MMO coating in not known.
MMO was inserted when Na Chloride concentration was 180 grams per liter and Na Chlorate concentation 240 grams per litre.
Current density on MMO was approx. 200mA per square cm. 2.2 litre cell with 8 Amps current from constant current supply.
Voltage accross cell varied from 4.3 Volts @ at start of MMO run to approx. 4.5 Volts (before Anode passivation). pH was
maintained at around neutral with acid addition required all the time. This contrasts with Graphite where the cell does not need
acid additions when coming up the the Perchlorate point. The temperature was around 60C.
CE figures are calculated by using Chlorate production as ascertained with titrations.
First 61 hours (492 Ah) CE was 65%. (Seems rather low.) Next 81 hours (652 Ah) CE was 55%. Next 39 hours (312 Ah) CE was 33%. The Na Chloride
concentration at this point was 21 grams per liter. There was no Perchlorate present.
Twenty four hours later the current was still flowing OK. Twenty hours later the Anode was discovered passivated with a test for Perchlorate being
positive. CE was not measured for the last 44 hours.
Moral of the story is don't be trying to make Perchlorate with LaserReds MMO! or any other type of MMO IMO.
It is probably not wise to be lowering the Chloride concentration of a Chlorate cell much below 100 grams per litre or perhaps 150g/l if you want to
keep CE high when using MMO.
At what point the MMO starts to wear or if that wear starts suddently at a certain point is not known.
It may be a bit like Graphite where large erosion starts fairly abruptly at approx. 30 grams per litre Na Chloride (pH controlled cell).
There is very little difference to see between the passivated part of the MMO Anode and the part that is not passivated at the top where the
connection was (outside the cell).
On a slightly different note. I tested my Lead Dioxide plating solution some days ago and it tested positive for Nitrites.
I added approx. 10ml 35% H2O2 into two litres of solution and left it sitting for approx. 4 days. When tested for Nitrites they
were gone so I guess H2O2 done the job.
Dann2
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Dann2, the anode just 'passivated', didnt some of the MMO layer flaked off?
Some experiments I did on high concentrated chloride solutions (NaCl, KCl) for chlorate, on low-to-moderate current density, revealed some almost
undetectable tiny black ppt that was not from solution (because when using MMO I always filter the solution prior to feed the cell with it) nor from
cathode (Ti will produce at best a tiny white cloud of TiO2). Maybe just a natural wear of laserred MMO anode.
Is good to see that this is able to produce perchlorate. Bad enough, the mother nature dont keep it to produce a decent amount, without being
destroyed.
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
@ Dann2
Awhile back there was some discussion about a high grade of aluminum as a cathode. Is this workable or a poor choice? I have been having a tough time
finding Ti and high grades of stainless has been a hit or miss thing. I have found that very fine stainless steel screen made in either the US or EU
seems to be copper free.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I think just all types of Aluminium will corrode. I don't recall it being discussed up the thread or anywhere else. I have never tried it myself.
Woulter Vissers page says it will corrode. Try just about any type of Stainless Steel. It should be OK. If you are going to get corrosion it will be
above the water line. You could perhaps try covering the part above the water line with plastic or epoxy, etc. Dont leave it in the cell when it is
turned off.
@AF100%
I cannot see any MMO flaking off. The MMO mesh is slighty less black than it was at the start. This cell had a mild steel Cathode in it for a while so
there is brown stuff (Fe compound) at the cell bottom which will hide any small amounts of MMO that may be there.
Swede ran this MMO in a Perchlorate cell for quite some time and the MMO Anode did not passivate. His cell had not much Chloride in it, a 'pure'
Perchlorate cell. (I cannot for the life of me find the account, can you help Swede?).
He did NOT get any Perchlorate to form BTW.
The MMO may be a bit like Pt. The absolute NO NO zone is where there is some Chloride still in the cell and the cell is starting to make Perchlorate.
Both Oxygen and Chlorine are being produced at this stage and this is bad for a Pt Anode and perhaps the same applies to MMO?
Dann2
[Edited on 24-2-2010 by dann2]
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Hello dann2
I dont know if this is feasible task or even a dumb question, but how about 'regenerating' at home fully used (passivated) MMO anodes like from
lassered with ruthenium chloride/ tetrabutil titanium (both from e.g. university lab) and a heat gun? Do you really need to completely etch the used
anode to put a new MMO layer or you could just decompose the salts on the old layer with good results?
If ones have acess to little Ru chloride I think this could be interesting to test..
[Edited on 24-2-2010 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Once the Ti has passivated it means the TiO2 is present and you will definitely have to etch the Ti to get rid of it.
Dann2
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Sorry, it seems was really a dumb question 
I just wondered if most of the MMO layer dont flake but rather suffer any kind of modification that dont allow it to conduct current, and if there was
any other way than etching to bring life again to the MMO.
One of my MMO laserred anode for example have some rusty spots on it (Ive used this same part of the mesh a couple of times) but keeps working. Wonder
what can be this.
[Edited on 25-2-2010 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Laserreds MMO is second hand (afaik) and will be of unknown quality. It may vary from shipment to shipment. Perhaps the stuff I have has a very thin
coat which is why it wore off so quickly.
Swede was cleaning up some of his Laserred MMO using HCl to remove the brown stuff.
Dann2
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
Probing into the great unknowns of a Chlorate cell (as one does) may be easier than thought based on Swedes higher Voltage=less Chloride observation.
I put together a circuit to work with my two Pt probes and it seems to work OK. I have only tried it on pure salt solution (cell start) and liquor
from a finished Chlorate cell (just at the Perchlorate point from the last MMO run).
The sample is placed into a glass U tube that is one cm inside diameter and 230 cm total length untill the tube is full. The probes are then placed
into the top ends of the U tube.
There is an advantage in cooling down the test solutions as you get a large 'signal' between the start and end conditions of cell run.
At 19°C the device gives a reading of 6.2 for start and 7.8 for end of run, a difference of 1.6.
At 0°C the device gives a reading of 7.4 for start and 9.6 for end, a difference of 2.2.
The Zero degreese is also easier to replicate as opposed to some arbitary temperature.
The circuit puts a constant current between probes. The probes are not exactly equal as I made a blunder when constructing them. One is 1 cm long Pt
by 0.3mm wire and the other is 0.5cm long my 0.3mm wire. Current into probes is approx. 37mA (steady). This gives a CD on probes of 785mA per square
cm and 392mA per square cm.
This high CD gives a better 'signal' as I tried lower CD and got less 'signal'.
I also tried different wave forms etc into porbes using a signal generator and a scope to see the results but the good old DC seems to be the best.
Also the use of a U tube gives a better signal as opposed to simply using a small container to hold the sample. A longer U tube may help more but you
will have to use a higher Voltage on the CC source which is 12V for my circuit. This can be run from the Computer power supply powering the actual
cell.
The actual Voltages read above from the probes are 1.18 * meter-readings Volts approx. as the meter in picture reads from 0 to 11.8 Volts (0 to 10 on
meter).
It may not be a proper solution to solving what stage a cell is at because if you are adding slops from previous runs this may complicate matters but
it seem to give a clear read out of a new cell from start to finish.
Dann2
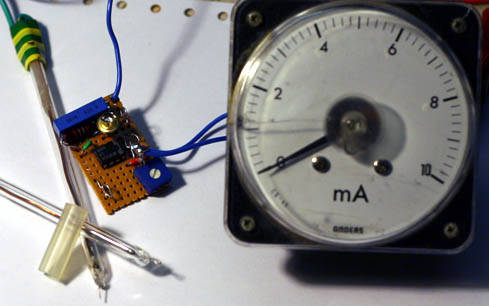 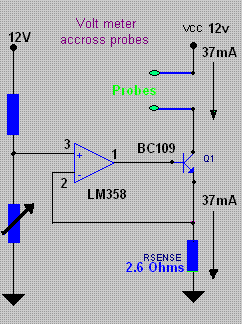
[Edited on 27-2-2010 by dann2]
|
|
|
WSM
Harmless

Posts: 5
Registered: 25-2-2010
Member Is Offline
Mood: No Mood
|
|
Hi Swede,
I was trying to reach you on APC but either I can't get there or it's gone 
 ! I really hope all your blogs are safe! Please E-mail me sometime or leave a
message here. Thanks, old friend. ! I really hope all your blogs are safe! Please E-mail me sometime or leave a
message here. Thanks, old friend.
WSM
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
Try this www.amateurpyro.com you will be happy
[Edited on 2-3-2010 by Bikemaster]
|
|
|
WSM
Harmless

Posts: 5
Registered: 25-2-2010
Member Is Offline
Mood: No Mood
|
|
Yes!!! Truly happy.  Thank you. Thank you.
WSM
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Thoughts on Cathodes
Hello,
I have been running a cell for the last 13 days or so to see if using a very large Cathode effects current efficiency (CE).
I started with a small Cathode for the first three days to let the cell stabilize so that acid additions would be steady.
A Graphite Anode was used. It was a cell I ran many times before. Current into cell was 4 Amps (constant current supply). Cell is 2.2 litre's in size.
Temperature was around 48C.
I got a CE of 47% for the first three days. Then I added the large Cathode made of mild Steel. It is very large with a total surface area of 714
square CM (counting front and back) and are coaxial to the Anode. (see picture above on 29 Jan 2010).
I got a CE for the first three days of 47%. CE is inclined to be low at cell start in my experience.
I let the cell run for a further 10.4 days and got a NEGATIVE ! CE.
Chlorate in cell at start of 10.4 day time run was 78.5 grams. Chlorate in cell at end of 10.4 days run was 59.4 grams (using titrations).
I checked my samples again as I thought I had made a mistake.
The cell is going in reverse.
KEEP CATHODES SMALL
I suppose if you are using additives to stop Cathodic reduction they it may not matter so much but with 'green' cells (no additives) it is very
important to keep Cathodes small.
The cell demanded acid at a rate above the normal (+17% more) in order to keep the pH at around neutral. I was also trying out the 'probes' (for
probing the great unknowns of all Chlorate cells throughout the land) with little success(or so it seemed). The Voltage was going the wrong way. I
guess they may be working OK after all.
Dann2
PS. Where is Swede? Hope he has not run foul of the Texas law, 'No beakers allowed........'
[Edited on 12-3-2010 by dann2]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Question
Experiment: 2 cells operating in similar circumstances
2.5 Liter cell KCl solution 320gr per L
2.25 L in both. One MMO anode / Ti cathode
The other: Gouging Rods both anode/cathode
Both exposed to 6A via computer PS for 13 days
Gouging Rod cell had better yield @ 169 gr / MMO cell 148 gr
* Could the graphite in the brine play a part in greater conductivity if current were that low?
In another situation large cell (5gal bucket filled 2.5 gal similar brine solution) MMO anode, Ti cathode 60A: Yield weight UNK - but much
greater than similar configuration with Graphite.
Smaller cell w/ lower current gave better results with graphite than MMO/Ti - Larger cell, higher current levels did much better with MMO/Ti - Water
temp in larger cell approx 50 C smaller cell - temp 60 C (+ or -)
Wear on rods similar in both; thus smaller cell had higher concentration of conductive material: conductivity of brine would change as time goes on.
Can the addition of a steady conducive material (graphite) in the brine make for higher efficiency w/ a concentrated environment such as the
smaller cell?
* Note that SOME tracking of Voltage was attempted (switching supply: voltage not constant). Generalized construct, Start 4.50Vdc, day 8 5.10Vdc
Finish 5.20Vdc in graphite cell. MMO cell, Start 4.25v Finish 5.85Vdc. Greater variation on MMO cell.
[Edited on 12-3-2010 by quicksilver]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Quicksilver,
The conductivity of the electrolyte has nothing to do with current efficiency.
What are the current densitys on the Anode materials that you are comparing?
Graphite works best around 34 - 44 mA per square cm.
MMO (I am not too sure actually) works around 200mA per square cm.(these are the figures industry uses)
Don't get too 'hung up' on the Voltage appearing accross the cell. It does not really tell you a whole lot. Most beginners (don't know if you are a
beginner) seem to take it into their heads that it's all about 'the Voltage accross the cell'.
If you want a lower Voltage accross the cell place the Anode and Cathode closer together. This will give greater power efficiency, which is not the
same thing as greater Current efficiency.
Regarding the Voltage accross cells, in extreme cases I have heard it argued like this:
The Voltage accross my cell is 4 Volts-------------> Therefor I am making Chlorate.
The Voltage accross my cell is 7 Volts-------------> Therefor I am making Perchlorate.
This is simply rubbish.
If you want to attempt comparing Anode materials think in terms of current density on Anodes and also have a similar cell-volume to current-into-cell
ratio for each comparison. Thats really the best you can do.
Graphite is a very good Chlorate maker. MMO is only slightly better with only a very small lower Voltage needed to get Chlorine to be evolved. These
small Voltage levels are levels that you WON'T be measureing unless you place a third electrode (reference electrode, satureted Calomel bla bla bla)
in the cell.
Dann2
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Quote: Originally posted by dann2  | Hello Quicksilver,
The conductivity of the electrolyte has nothing to do with current efficiency.
What are the current densitys on the Anode materials that you are comparing?
*************snipped for brevity*********
Dann2
|
Hello Dann2:
Thank you for your reply.
The info:
"Graphite works best around 34 - 44 mA per square cm.
MMO (I am not too sure actually) works around 200mA per square cm.(these are the figures industry uses)"
was quite valuable.
I was comparing MMO & Graphite - current: same, MMO of the perforated commercial type available via eBay 2x6". Rods were 5/8" diameter and contact
with brine 8" depth. Due to the perforations I had no way to really assess the total of area in contact. Measurement was with a DIMM as I had no "amp
clamp" at that time but it popped up at 6amps on both Computer PS held steady but when I did manage to borrow an amp meter they had both held at 6.12-
6.36a. but I had to do one after another. There were many flaws in my comparison. But when I weighed the yield on the 2 liter cells I did so when dry.
{The larger cell was a visual comparison & for the most part valueless as data.}
Illustrating the possibility of the defused conductive particulate within the brine as a boon to yield.
You answered my query. Generally because of the resultant I had thought that the conductivity of the graphite that there MAY be an advantage to the
hassle and time spent in clean-up of this black mess :-)
When I was quite young and children had "shop" classes in school & could work with "dangerous" things (& learn to love science etc.) I had
been told that if one could obtain "pure" water that there would be no conductivity but rather it was the materials with water that would conduct
electricity. There I had thought that if one had a STABLE conductor within water (the brine 's conductivity must change as the electro-chemical action
takes place) that there MAY be a slight advantage to it's efficiency.
That my yield was slightly greater with a graphite anode gave rise to this question. What's more, I have no logical premise to base the fact that my
yield was slightly better. I could go wild & guess that slight differences in anode-cathode separation could account for this or that water temp
played a part - but I have no answer.
I DO take notes however my recording would need to be much more meticulous for me to do anything but grasp at straws. The fact this this occurred in
ONE experiment leads me to believe I have no real basis for any belief in that direction. If it occurred in four or more straight shots in a
row....& my accuracy was much keener than it had been - perhaps then I could develop a premise that there is some superiority in conductive
particulate within the water making a difference.
The fact that industry does NOT use graphite punches holes in that.
However the loss of time in processing, disposal of waste, extra steps could make such a thing (IF possible) of little importance. Even the gain of
10-12% consistently would be outweighed by such an expense.
Of interest was when you used the statement "keep cathodes small" - by what percentage? Many if not most cells appear to have same size or in some
cases much larger Cathodes.
[Edited on 12-3-2010 by quicksilver]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Quicksilver,
There is a page here:
http://www.oxidizing.110mb.com/chlorate/chlorate.html
that may help expain the chemsitry going on in the cell. Perhaps you seen it before.
I find the best way to get rid of the Graphite powder is to give the finished electrolyte a good boil to get rid of dissolved gasses and let the
contents sit for a few days. All the Graphite goes to the bottom and you can siphon or decant off the 100% clear liquid and leave a small amount of
black liquid behind. You can filter this small amount of liquid if you so wish. This will not work for K Chlorate only Na Chlorate.
If you don't boil the cell contents the gas seems to keep bubbling up and keeps cell agitated and thus keeps Graphite in suspension.
Stupidly large Cathodes are a disaster if you do not have additives in the cell (Chromate etc) to stop reactions at the Cathodes turning wanted
products back to Chloride. Try to keep total 'active' Cathode area similar to Anode area or perhaps better down to 50% of Anode area. I say 'active'
area to distinguish between the side of the Cathodes facing the Anode and the 'inactive' side which is the side away from the Anode. It would be great
to cover the 'inactive' side with plastic but perhaps that's being too exacting. The small Cathodes will give a higher Voltage accross cell but that's
no big deal IMO.
In my opinion there should be two Cathodes for flat Anodes, one each side so that the current on the Anode is distributed fairly evenly. Use three or
four for rods.
I used to think that a great way to make a cell would be to obtain a large SS bucket and simply suspend an Anode in it. The bucket would be the
Cathode. This would be a very very bad design if you had no additives and it might even make NO Chlorate.
Industry (monopolar cells) has equal Anode and Cathode area in Chlorate cells and they do not have much 'inactive' Cathode area because the
Anodes/Cathodes are usually arranged in banks. The relatively small 'inactive' areas of Cathodes (at the ends of banks) may (guess) be covered to stop
small currents leaving the surface. In bipolar cells it's not an issue at all.
The current density on the 'inactive' surface (the surface not facing Anode) will always be quite low. It is low current density areas on the Cathodes
that are commiting the sins (read in Ullman) so there may always be a small advantage in covering 'inactive' areas even if Cathodes are relatively
small.
Most cells that you see (amateur) may have a Cathode the 'same' size and the Anode on each side of the Anode. But that a total of twice the area of
the Anode if you count the (sinning) 'inactive' side. Most of us don't like adding additives.
Just one note. The Cathode I used above was mild steel that was well rusted. This is the worst type of material for converting products back to
Chloride. Ti would not be as bad but I don't know how much better it would be. You are not going to use big monster Ti Cathodes anyways.$$$
Dann2
[Edited on 12-3-2010 by dann2]
|
|
|
| Pages:
1
..
33
34
35
36
37
..
48 |