| Pages:
1
..
31
32
33
34
35
..
60 |
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
Yeah! But it must have been a lot of trouble to stand the smell, so it's equally difficult 
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
I don't know as most of us necessarily have better equipment than Brandt... he probably had a pretty decent iron retort and lots of fuel. Also, he
didn't cook 'his own piss', he had multiple thousands of liters from the people of whatever town he was working in (as I recall).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
It’s only funny if you look at it superficially, less so IMO if you know the story better.
Brand spent large amounts of money on scientific pursuits, without much success, throughout his life, gradually getting his family into debt.
With his ‘urine cooking experiment’ he was actually trying to make gold, which at the time was believed by some to be contained in urine (hence
the colour!). Hence the concentrating of much piss, and the subsequent attempt at reducing the ‘piss concentrate’. The look on his face when he
didn’t produce gold but obtained the first man made phosphorescing material ever, must have been priceless.
Brand later spent much of his life trying to sell his method (he kept the details secret for quite some time) for making phosphorus to leading
scientific figures of his time but only succeeded a few times.
The reason why most of us here only manage to make small amounts is mainly: resources. Brand’s furnace, also going by the picture below, was likely
to be quite large and charcoal fired (= very hot):
http://www.google.co.uk/imgres?imgurl=http://www.bpc.edu/mat...
[Edited on 2-7-2011 by blogfast25]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | | With his ‘urine cooking experiment’ he was actually trying to make gold, which at the time was believed by some to be contained in urine (hence
the colour!) |
<iframe sandbox width="640" height="510" src="http://www.youtube.com/embed/TkZFuKHXa7w" frameborder="0" allowfullscreen></iframe>
[Edited on 26-7-2011 by peach]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Very funny. I thought I had seen all of Blackadder but I don't recall the 'green' discovery!
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
sooooooo. i had a go at turning some of my caucasian phosphorus into native american phosphorus. it seems that it is not too hard. i placed about 2
grams into this vial and put it in the sun where it would not be disturbed.
i have noted some properties that may or may not be of interest. the P4 undergoes change rather quickly but surface area is quite key. it is quite
warm here (around 38-40 celsius) and so, of course, the P4 liquefies in the vial since it is against a surface that gets a good deal warmer than this.
this is not so odd but the phosphorus exhibits the properties of a supercooled liquid in that it will not solidify until it has been cooled
significantly and agitated.
there also seems to be some level of sublimation. as you can see from the photo, the entire inside of the tube is coated with a deposit of red P.
as far as surface area, this is difficult to control with a liquid in such a small, closed container and extensive handling of the starting material
is arguably dangerous. i have scraped the sides of the vial when i bring it inside at night and by the end of the following day, i find the vial
coated again with the red deposit. much repeating of this will eventually convert most of the material but a larger vessel, allowing more contact to
more of the product by solar radiation will undoubtedly push the conversion along faster. just wanted to share what i have been up to lately. i will
get back to work on the mixed amine synthesis very soon.

|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
This is a bit offtopic, because it's not a preparation from a compound per se, but the thread on micropreparation is not so appropriate, and opening a
new thread has no point.
I wanted to make a sample of white phosphorus for my element collection, so I started doing some small scale distillations from red allotrope in
various self-made glass retorts, just to see what can go wrong before I try to do it on a larger scale. By larger, I mean in larger glass tubing.
My best advice to anyone trying to do this is - do not try it using flasks. Do it in disposable glass apparatus made out of glass tubing (not less
than 4 mm in diameter).
I find that designs like this one:
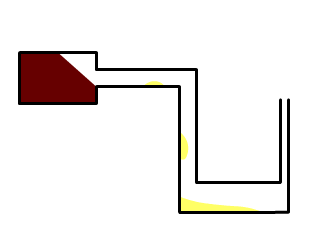
are the best, and if you make several such small retorts, you can make a reasonably large amount of the substance in less than half of an interesting
afternoon. If someone wants me to explain the flameworking part, let me know.
I loaded the tube with the red P before finishing the last narrow part.
The retort is meant to be held in hand, using wooden tongs or similar tool. The globules of the molten phosphorus collect in the first horizontal
part, and when they become larger than 2-3 mm, you incline the apparatus and they slide in the lower part. There absolutely has to be a vent at the
end, and the lower part must be wide enough so the phosphorus won't make a plug. If it does, either the reaction vessel will burst (and then you're in
danger) or there'll be some fireworks at the vent tip, which are benign if the vent is pointed away from you. Either way, this means you have to wear
protective equipment.
I've tried to distill it directly in cold water, but sooner or later, either water or air will find their way into the hot retort. P will catch fire,
or the retort will crack, and I'll leave to your imagination what can come out of that situation. It is possible, it is quick, but it's also nerve
wrecking, because you hold the retort in one hand and cold water in another.
The key is to heat the red P as less as possible, because the more you heat it, the less nice and transparent globules you get.
Patience is needed to collect very pure white P, because extra heat turns makes it orange. I've managed to make nearly pure transparent P, but I
smashed the retort to see it glow. 
Start with very small amounts, few matchhead-sized pieces at first, and practice manipulating and ampouling the phosphorus. You can make the first
matches and smash them outside during the night.
After the proper distillation, you'll get lots of solid blobs which can be joined together by gently heating the water over 45 °C. Never remove the
phosphorus from warm water, even if it's solid. It's seconds away from catching fire.
Used retorts contain some white P, so be sure to destroy them appropriately as they're are a fire hazard.
One cool thing is that white P, or at least the impure one I got seems to be capable of undercooling. It will stay molten under water at room
temperature until you touch it with a glass rod.
This transparent yellow sample (not under water, just ampouled) is still molten, and it's around 28 °C in my room.

The glow is just beautiful. 

Unfortunately, I don't have the equipment to take a nice long exposure macro shot.
It's eerie, like a liquid light with a greenish tinge that flutters lightly when you try to blow it. The less orange the P is, the more it glows. The
piece was increasing its glow, so I returned it to water immediately.
[Edited on 7-8-2011 by Endimion17]
[Edited on 7-8-2011 by Endimion17]
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
It seems we all ignored one thing... An experiment I did earlier was very effective. It was not to produce phosphorus, but it inspired me to think of
this:
No one mentioned acid catalyzation. Am I ignoring something? Am I forgetting that all phosphates blow up in acids? What if we can somehow use acids to
react all the compounds necessary and eliminate the need for distillation, furnaces, etc? Could we use... say... acid catalysation along with reflux?
What if we take some TSP, add it to an RBF along with carbon and sand, pour in some water and acid, reflux, and call it a day (after we get
phosphorus!)?
Just an idea. Don't crucify me if I am ignoring something...
EDIT: BTW good job to the previous poster on the glow in the dark ampoule worthy desk nicknack  !! !!
[Edited on 8-8-2011 by ScienceHideout]
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
i am pretty certain that this method, along with electrolyzing phosphate solutions and reduction of phosphoric acid in a microwave have all been
discussed in depth in this thread. if, however, you want to give it a go, i am sure there would be many parties interested in your results. just be
sure whatever you use, that any phosphorus produced will not react with the chemicals used. it is quite reactive.
@ Endimion17, woelen has the most fantastic site with some killer projects he has performed and outlined in great detail with pictures and even some
videos. he has one that is similar to the distillation you performed. you should have a look <a
href="http://woelen.homescience.net/science/chem/exps/RedP2WhiteP/index.html" onclick="window.open(this.href); return false;">here</a>. have
a run through the rest of his site as well. it is truly amazing.
good work on the WP. i noted the supercooling properties as well. that makes WP even more dangerous than anyone first thought as some of us have
observed that phosphorus in liquid form at any temp will ignite instantly when exposed to oxygen. as a solid i have noticed it is tame until oxidation
raises its surface temp to the melting point.
[Edited on 8-8-2011 by Rogeryermaw]
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
@ScienceHideout: Your idea reminds me of experiments like making potassium "the other way around". You should check the enthalpies etc., and do lots
of microscale experiments. There's no definitive answer to your question, and if you find a forgotten/unknown way of producing P, you could patent it,
and if it's efficient, earn some money. Of course, you understand there's a very small chance for that.
There's a god reason why factories make P using lots of energy, and it's not someone's wish. If they knew a more efficient process (and there are
scientists working on it) they'd use it sooner or later.
@Rogeryermaw: I know about his site, it's truly a great one, but I don't like the vessel he used for phosphorus. If I recall correctly, it was a
poorly bent test tube in which large amount of WP was collected. Those things have a tendency to crack if they weren't annealed, and you really don't
want that, as it will lead to a spitting inferno. "May lead" and other legal minced oath BS is not appropriate language here.
And the whole idea of working underwater, cracking the tube, filtering and manipulating this hideously poisonous pyrophoric substance is not something
which makes me happy, as I don't really like heavy protection equipment and really like my precious liver.
This apparatus
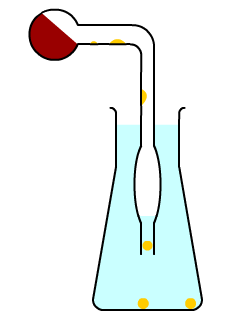
when small enough, can be safe if one knows how to handle it. My vessel was <1cm in diameter. Spherical blobs of WP form while falling trough the
upper tube, and the widened part can be useful for enhanced cooling and avoiding a quick sucking back of the water. That problem is actually rather
avoided by making a proper retort with a small reaction vessel, relative to its neck, which avoids the thermometer-like behaviour.
Once you start the process, you should finish it.
During the first few minutes, vapors (WP, pentoxide) and some small bits of WP will exit the tube and self ignite in air after bubbling through the
water. They produce popping sounds, like hydrogen in a test tube. It's annoying and somewhat scary, but benign (small scale!).
Also, the pentoxide vapors will try to "suck" the water in, so be careful not to stop heating at that moment.
Regarding the heating source, it might be possible to do this with a candle. That's how energy intensive this is.
It's best to heat the tip of the vessel, to avoid passive heating of the neck as much as possible.
And if you make several small retorts, the danger is very small, and you get the joy of distilling red P several times. 
It's elegant, fun and requires small energy input. And you can make almost transparent and white WP if you're careful not to overheat, and to cool the
blobs immediately upon forming.
The liquid WP in my ampoule stayed liquid untill night, when I started messing with it. It touched a rough point and turned solid. I estimate the
speed was around 2 m/s, as I saw the wave of solidification. It gave small amounts of heat at that point.
[Edited on 8-8-2011 by Endimion17]
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
 Thanks for your reply. I will definately try some microscale... but I think
everything would be better if we all just distilled 60 buckets of pee with some sand and carbon. Thanks for your reply. I will definately try some microscale... but I think
everything would be better if we all just distilled 60 buckets of pee with some sand and carbon.
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
brandt's experiment would be much more productive if one skipped the whole putrefied urine step and just ordered some microcosmic salt. it's quite
cheap. i think the toxicity of WP is serious, but it does not scare me. its pyrophoric properties are much scarier to me, having been burned by it
before. the wounds heal slowly and are excruciatingly painful.
woelen's bent test tube is a bit crude and there is room for improvement, but the ingenuity he shows is inspirational. as for me, i doubt i will ever
distill red phosphorus. it is too simple for me to run the furnace, which, of course, is done outdoors. i made quite a bit of WP last year and still
have enough that any runs in the future are solely meant to streamline the process and try to improve yields.
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
What about those aqua globe retorts shown to youtube by chemical myst?
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Here is the link to the video he refers to. <a href="http://www.youtube.com/watch?v=hDpVEX5SZzg">myst32YT's 10 useful tips.</a>
It's a great video, and a good channel.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Here, proof I've made some really white P.
It's very hard, but it's possible.

Those are fresh blobs together with the piece I've shown earlier.
Only the first few fresh droplets are white, after that, they're getting yellow, then orange. Temperature of the retort is not relevant, I've checked
that by halting the process for a few minutes.
It's like something happens in the red P. Maybe some kind of reaction between nonvolatile residues in it, or between fresh WP and red one, I don't
know.
In order to get lots of really white WP, one would need to slowly heat lots of pure red P and immediately cool the vapors. Afterwards, yellow version
distills.
Impurities in the retort shift it towards orange.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Is this the first photo on teh internets of glowing WP? 
Man, it's hard to take those photos with a crappy camera, though I've enjoyed the supermacro option.
I've tried to do it with a small blob, but it caught fire after it melted. I've got it all on video, which was pure luck as the thing allows only 10s
videos at a time. Weird glowing and then wham, fire, right in third second. Stay tuned, there'll be an update at this channel.
The worst part is when you have to set the camera, and the thing just partially melts. You try to pick it up to put in in the water, but it's stuck to
the paper, and behaves like tree resin in the summer. You throw the bigger piece in the water, and the leftover sits there stucked, seconds away from
bursting into flames. 
|
|
|
Damitwhy
Harmless

Posts: 8
Registered: 14-8-2011
Member Is Offline
Mood: No Mood
|
|
I've been very interested in building a furnace and "do it the old-fashioned way" myself but I realize it would be so much easier, less time
consuming and more manageable if I were to use an "Induction Heater Coil" as the heating source with a Steel or Iron Retort.
This link shows a home made induction heater coil heating metals to red/whiteness with in seconds :
http://www.youtube.com/watch?v=LT5VvBQfOoo&feature=relat...
Costs for purchasing some thing like this would be in the thousands but this other link shows affordable home build:
http://www.richieburnett.co.uk/indheat.html
I've got less money than sense but would love to give this a go... if any one else gets there before me please give feed back and tell me how you got
on 
Attachment: Phosphorus.pdf (527kB)
This file has been downloaded 791 times
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I'm kind of surprised no one has brought up induction coils in this context before. It's not without its own problems of course but it's definitely a
'21st Century approach'. Nice work if you can get it and don't get shocked!
|
|
|
Damitwhy
Harmless

Posts: 8
Registered: 14-8-2011
Member Is Offline
Mood: No Mood
|
|
Wow its not as costly or even much hassle when an induction hob can be purchased for less than £50... Stumbled across this induction hob and realized
its potential not only for the phosphorus furness (with modification) but in reflux heating if ceramic coated iron pellets were used as boiling
chips...
http://www.mirrorreaderoffers.co.uk/item-pp-d3197/induction-...
With a little modification and reverse engineering items like this could be in most hobby chemist work places 
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
Those only work well for large pans with thinly clad layers of copper- For lab use we'd need something with a very different induction value (and much
higher frequency).
|
|
|
vr6t4dr
Harmless

Posts: 2
Registered: 23-8-2011
Member Is Offline
Mood: No Mood
|
|
Phosphorus sesquisulfide
so, is there any way to use Phosphorus sesquisulfide?
|
|
|
Damitwhy
Harmless

Posts: 8
Registered: 14-8-2011
Member Is Offline
Mood: No Mood
|
|
I'm not sure, I've not found anything via google searches but I'm pretty sure that if you were working with a pure dry sample of Phosphorus
Sesquisulfide you could still make the reagent Phosphorus Trichloride (PCL3) with Sulphur Dichloride as a side product by direct Freeradical
Chlorination in Dichloromethane (DCM)...
this great youtube link will explain how just by substituting pure Sulphur for Phosphorus Sesquisulfide... Not completely sure though so Dont Do This
without doing your own research into this reaction..!
http://www.youtube.com/watch?v=HTzUhLuYlHU&list=FLLQ41kT...
I suppose this post was pretty unrelated but I thought it would be interesting just jumping straight in with a reagent most people would need pure
Phosphorus for...
|
|
|
1911
Harmless

Posts: 13
Registered: 29-6-2011
Location: Finland
Member Is Offline
Mood: No Mood
|
|
There was somewhere in this thread a mention that elemental phosphorus can be made by reacting phosphine with copper(II)chloride (solution) in
following reaction: 4 PH3 + 6 CuCl2 --> P4 + 6 Cu + 12 HCl. This could be a superior method over the retort method as it could be done using glass
apparatus. Am I now over looking something why this wouldn't work of why this method hasn't been discussed before?
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 1911  | | There was somewhere in this thread a mention that elemental phosphorus can be made by reacting phosphine with copper(II)chloride (solution) in
following reaction: 4 PH3 + 6 CuCl2 --> P4 + 6 Cu + 12 HCl. This could be a superior method over the retort method as it could be done using glass
apparatus. Am I now over looking something why this wouldn't work of why this method hasn't been discussed before? |
likely due in large part to the extreme toxicity of phosphine. it is a gas at s.t.p. which makes it difficult to handle for the average home
experimenter. as part of the process i have used to synthesize white phosphorus from 6(Na(PO3) it is generated in small quantities with diphosphine
which causes it to ignite instantly on contact with oxygen.
do not forget that metal phosphides are used as powerful rodentacides in farming applications. metal phosphide is reacted with water to produce
phosphine gas which is quite deadly.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 1911  | | Am I now over looking something why this wouldn't work of why this method hasn't been discussed before? |
Phosphine toxicity. As mentioned by Roger. Don't go there as a hobbyist...
|
|
|
| Pages:
1
..
31
32
33
34
35
..
60 |