| Pages:
1
..
30
31
32
33
34
..
77 |
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
Photos from my internship:
What the guy beside me do :

What i do while he sacrifices virgins :

-_-'
[Edited on 16-5-2015 by alexleyenda]
Help us build the Sciencemadness Wiki! Every question and tips about amateur chemistry two clicks away, wouldn't that be awesome?!
sciencemadness.org/smwiki
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|
It's a tough call as to who has the better job...
They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
When fermenting berries in the lab, always strain the mash first!
|
|
|
Zombie
Forum Hillbilly
    
Posts: 1700
Registered: 13-1-2015
Location: Florida PanHandle
Member Is Offline
Mood: I just don't know...
|
|

They tried to have me "put to sleep" so I came back to return the favor.
Zom.
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
^^By the way, the red stuff was a kind of Iron 2+ complex with a bidental triple cycle ligand, I forgot it's name, anyway it's not my project as I
said :p
Help us build the Sciencemadness Wiki! Every question and tips about amateur chemistry two clicks away, wouldn't that be awesome?!
sciencemadness.org/smwiki
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by MrHomeScientist  | | I've tried that one and it yields Bi powder, which isn't very interesting to me so I tried melting it down to an ingot. That failed, and instead it
oxidized to yellow bismuth trioxide! Too finely powdered, I guess. |
Yeah, I had the same experience! Even
when I tried to melt it under argon (using a wine preservation canister). Oh well. It isn't very pretty for the element collection but at least it
still works for chemistry.
|
|
|
Gleb
Harmless

Posts: 8
Registered: 23-4-2015
Location: Russia
Member Is Offline
Mood: No Mood
|
|
My collection (others)
1. Mn(SO4)2
2. naphthalene
3. thiourea
4. Na2B4O7
5. K2SO4*NiSO4
6. Bi
  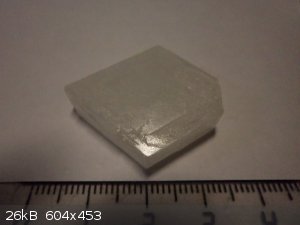   
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Nice flask there Alexleyenda. Love the column too. I learned a lot doing an internship like program hope you did too.
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
Gleb - Beautiful work, there!! I aspire to grow my own as well as yours. Can you tell me anything about the potassium sulfate and nickel sulfate
crystal in your last picture?
Nizzo - Nice Nd sulfate crystals! How do you prevent yours from cracking? My medium sized ones (about that size) like to split along planes.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
<img src=http://i.imgur.com/vGCOVMu.jpg width=800>
<img src=http://i.imgur.com/cCwBiC6.jpg width=800>
The same holmium citrate solution, in tube lighting and CFL respectively.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Gleb
Harmless

Posts: 8
Registered: 23-4-2015
Location: Russia
Member Is Offline
Mood: No Mood
|
|
Wizzard, thank you!
I crystallized the stoichiometric amount of nickel sulfate solution and potassium:
NiSO4+K2SO4+6H2O=K2SO4*NiSO4*6H2O (blue-green crystals)
temperature=20 C
quite fine chemicals
isometric evaporation method
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
<img src=http://i.imgur.com/By8k73e.jpg width=800>
Here's some of the holmium citrate solution I'm drying, under CFL light. The above pictures aren't very accurate, as there still was a bunch of
particulate metal pieces that hadn't fully dissolved yet.
Here are the crystals that I've already made:
<img src=http://i.imgur.com/1gJSk0a.jpg width=800>
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Cool! Very small, too bad. How much holmium do you own?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
And there was B&F saying he had no Lab ...
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Probably has already been done but rhodamine B in water:
[Edited on 28-5-2015 by greenlight]

[Edited on 28-5-2015 by greenlight]

|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by greenlight  | Probably has already been done but rhodamine B in water:
[Edited on 28-5-2015 by greenlight]
[Edited on 28-5-2015 by greenlight] |
Looks similar to phenolphtalein cristals falling in NaOH solution 
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Yeah. But, heck, phenolphthalein crystals? I only've ever had solutions of it :/
Did you make the Rhodamine, or purchase it?
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
@ volatile chemist;
I purchased the Rhodamine online.
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Again; may have already been posted but some nice coloured smoke compos : :
 
[Edited on 3-6-2015 by greenlight]

[Edited on 3-6-2015 by greenlight]
[Edited on 3-6-2015 by greenlight]

[Edited on 3-6-2015 by greenlight]
|
|
|
DutchChemistryBox
Hazard to Self
 
Posts: 74
Registered: 24-3-2013
Location: Strasbourg
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by greenlight  | Again; may have already been posted but some nice coloured smoke compos : :
[Edited on 3-6-2015 by greenlight]
[Edited on 3-6-2015 by greenlight]
[Edited on 3-6-2015 by greenlight]
[Edited on 3-6-2015 by greenlight] |
Wow beautiful!
Can you tell us something about your compositions?
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
They are 50:30:20 Powder dye/Potassium chlorate/Lactose powder.
50 grams each wrapped in paper and tightly taped
Top left = Blue dye Top right = Purple dye (Rhodamine B)
Middle = Yellow solvent dye
Bottom = 50:50 Blue/yellow
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Cool, they look nice! Very opaque.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Could you elaborate more on what the blue and yellow dyes consist of?
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
Yes, please. I would like to know that as well. They look really nice!
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
I agree !
Today, in my internship, I made a zipper reaction, that is the result of ethylenediamine deprotonated by NaH, it becomes a nice purple !
 
Help us build the Sciencemadness Wiki! Every question and tips about amateur chemistry two clicks away, wouldn't that be awesome?!
sciencemadness.org/smwiki
|
|
|
| Pages:
1
..
30
31
32
33
34
..
77 |