| Pages:
1
..
29
30
31
32
33
..
60 |
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
It probably is. If it is really read, then it must be very old material.
Cleaning white P can be done by immersing it in a cold acidic solution of K2Cr2O7 of low concentration (take 0.5 grams, dissolve in 50 ml warm water
and add 1 ml of H2SO4 and then add the white P). Assure that the liquid becomes hand-warm, such that the white P does not melt.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Are you sure? Is the red P attacked by acidic dichromate and white P is not?
Why can't I allow it to melt? Because it will be contaminated with chromium?
I will do this when I get home.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
White phosphorus does indeed gradually convert to red P. Takes quite a while at RT, I'd imagine...
|
|
|
hkparker
National Hazard
   
Posts: 601
Registered: 15-10-2010
Location: California, United States
Member Is Offline
Mood: No Mood
|
|
Hey so I have phosphorus question, and I figured I could post it here without starting a new thread. Sorry if this has been adressed earlier and I
couldn't find it.
Matchbox strikers contain red P. Do you think I could distill it out? I would heat it in a test tube and the red P would boil off as white P, which
I would collect by leading the fumes from the tube into a beaker of cold water. If not, perhaps I could dissolve it out with CS2.
My YouTube Channel
"Nothing is too wonderful to be true if it be consistent with the laws of nature." -Michael Faraday
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
They contain exceedingly small quantities of red P. Yes, if all you want is a small quantity of phosphorus, you could probably remove the strikers
from several hundred matchbooks/boxes and extract it. It's not a promising approach for any sort of scaling up.
|
|
|
hkparker
National Hazard
   
Posts: 601
Registered: 15-10-2010
Location: California, United States
Member Is Offline
Mood: No Mood
|
|
Ok, thank you. I'm looking for a more OTC approach, but ill probably have to make it. Has someone made a comprehensive review of our progress here
so far like woelen did for the potassium thread?
My YouTube Channel
"Nothing is too wonderful to be true if it be consistent with the laws of nature." -Michael Faraday
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|
you probably want to clean it before distilling the P4. there will be glue mixed in with it as removed from the strikers. perhaps an acetone wash
would dissolve the glue but i am uncertain as to what solvent will work best.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  | It probably is. If it is really read, then it must be very old material.
Cleaning white P can be done by immersing it in a cold acidic solution of K2Cr2O7 of low concentration (take 0.5 grams, dissolve in 50 ml warm water
and add 1 ml of H2SO4 and then add the white P). Assure that the liquid becomes hand-warm, such that the white P does not melt.
|
I did exactly what you described. Most red material is removed this way, but still some red material is not removed. But it ok for my purposes. I use
the white P for small experiments (so reactions with other elements and such, and ofcourse burning the stuff  ). ).
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I was thinking of making a Phosphorus run sometime soon and I had a question.
I was going to make the flask out of ceramic but was going to connect to that with a copper tubing until I realized it may pose a problem by forming
Copper Phosphide. Is this a major concern or no?
After the fact I wanted to convert it to Red phosphorus by bottling it up and setting it out in the sun for a long time. I figured it would be good to
place it in an organic solvent and I know CS2 is perfered but its not avalible. What would be the best UV transparent solvent to use. It does not have
to solve alot of phosphorus just enough to keep the surface clean instead of having Red P form on the surface slowing down the conversion.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sedit  | I was thinking of making a Phosphorus run sometime soon and I had a question.
I was going to make the flask out of ceramic but was going to connect to that with a copper tubing until I realized it may pose a problem by forming
Copper Phosphide. Is this a major concern or no?
After the fact I wanted to convert it to Red phosphorus by bottling it up and setting it out in the sun for a long time. I figured it would be good to
place it in an organic solvent and I know CS2 is perfered but its not avalible. What would be the best UV transparent solvent to use. It does not have
to solve alot of phosphorus just enough to keep the surface clean instead of having Red P form on the surface slowing down the conversion.
|
Copper phosphide? I think that needs much higher temperatures to form. Using a copper tube as a collector may cause some mild attack on the copper but
not much to worry about, I’m pretty sure of it…
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Thats exactly what I was thinking as well. At the most I would worry about it reacting slightly which I was thinking may do something akin to
passification so it wasn't to much of a concern. I just didn't want to go on a hunch just to have it fail in the middle spraying Phosphorus fumes all
over the place.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sedit  | | Thats exactly what I was thinking as well. At the most I would worry about it reacting slightly which I was thinking may do something akin to
passification so it wasn't to much of a concern. I just didn't want to go on a hunch just to have it fail in the middle spraying Phosphorus fumes all
over the place. |
The chances of 'spraying' P all over the show are pretty slim: this is not a fast reaction, even at red heat. As the experience of this whole thread
shows, the trick is to keep the P hot enough without solidifying prematurely.
I believe that much can still be gleaned from the oil painting of Hennig Brand’s discovery of phosphorus:
http://www.google.co.uk/imgres?imgurl=http://mcclellan.yolas...
Of course the picture must have been somewhat ‘airbrushed’ but won’t be that far removed from Brand’s actual experimental set up…
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I got exciting news, for me anyway
I made another incarnation of the tube furnace from this thread,
http://www.sciencemadness.org/talk/viewthread.php?tid=9705&a...
(I will try to retrieve them pictures BTW they where on my website that got taken down.)
Im using the formula from page one given by Polverone using Al/ SiO2 / Metaphosphate. I placed the reactants into an old Iron Tube with the bottom
sealed with a cap. Once the furnace reached 900 degrees C I placed the tube (which had the top open so I could watch) into the furnace. It took about
5 minutes before I started to hear a crackling noise. Within 10 minutes or so the melt was fluid and like clock work every second there was a pop due
to P4 vapors igniting. Now all thats left to do is add a couple elbows and some more pipe to lead these vapors into water.
This is great because it means that anyone with a hair dryer, some old Iron pipe and creativity will be able to make Phosphorus quickly. The only
limitation will be the size of the pipe your using and how much reactants it will hold. When I get time I am going to pretty it all up and make it
self contained so that all I will have to do is add the reactants and plug it in. Within a matter of minutes start collecting the Phosphorus vapors
comming off.
How come I don't see anyone else using Nichrome to perform this reaction? It seems like the obvious choice here. Also I think instead of Silica flour
being used one could possibly get away with using fiberglass since it in itself is just very fine silica anyway the only difference is its in a thread
and not a powder.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Glass is, very simply put, sodium silicate. At high temperatures Al powder will reduce the disodium oxide in it. But it's worth trying...
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
No glass is Silica with various fluxes added, water glass is sodium silicate but I do agree and its something I was thinking about where using a
sodium silicate mixture to seal up a retort earlier. It doesn't really matter because I need to go pick up some 345mesh SiO2 for my ceramic work
Saturday anyway so thats no problem at all. I think they sell Magnesium powder there as well so I may just pick that up and use it instead. Sheesh I
love being into ceramics the list of over the counter chemicals becomes many factors larger then it normally is. I may even pick up a new roll of
Nichrome while im there so I can do this the right way.
I can't wait to give this a larger run tommorow its now to late for me to make a new furnace. Im making one with a 2" opening and inside 1.5" diameter
reaction vessle slides in. I already got the pipe work setup for it and its looking good but tommorow I have to setup the elements and insulation
around the new form so it can fit the new setup. I estimate I should be able to produce atlest a few grams of Phosphorus per run to say the lest but I
have no clue how long it will take per run to run it dry. It seems like it could run at a pretty good rate as it heats up but I did not want to let it
run to long because I could taste the Phosphorus in my mouth while working with it. It taste like Copper.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by hkparker  | | Ok, thank you. I'm looking for a more OTC approach, but ill probably have to make it. Has someone made a comprehensive review of our progress here
so far like woelen did for the potassium thread? |
Probably the most OTC approach is this:
1) Get a bunch of bones, preferably from pigs or cows, but chicken bones will do in a pinch, you just need more.
2) Heat these in your oven at its highest setting for like two hours.
3) Grind the bones into a powder, pretending you're a medieval alchemist. This powder is a mixture of carbon and calcium phosphate, mostly.
4) It's probably not quite enough carbon though, so grind up some lump charcoal, about half the volume of your bone powder.
5) Get some white sand and measure out about half the volume of your carbon. Mix the powdered bones, charcoal, and sand together very thoroughly.
6) Get one of those short pieces of cast iron pipe with threads on both ends, that looks like it'll hold all your powder.
7) While you're at the hardware store pick up some steel brake line. Also, get two cast iron caps that fit your pipe. Try not to look like you plan
on making a pipe bomb, although that is kinda what it'll look like. Try and make it wider rather than longer since that'll be easier to heat.
8) Drill a hole in one of the caps such that you can barely force one end of the steel brake line in it.
9) Get a coffee can, and line it with fiberglass (ok) or rockwool (best). Set your pipe bomb thing in there so the insulation comes up to just below
the top pipe cap. Then move some of the insulation out of the way so you can aim a torch so it hits the pipe and not the cap.
10) Get a flask half full of distilled water. Put it next to the coffee can and bend the brake line so its end is near the bottom of the flask.
11) Get your torch and aim it at the spot on the pipe. This isn't pressurized so it's not going to explode, but still be careful.
12) Heat it until bubbles start coming out the brake line, and watch for a white residue to form. Once new white residue stops forming, turn off the
torch. Pour out the water. There you have it, elemental phosphorus!
I think the reason more people don't use nichrome wire is a) you can't use it with metal vessels since touching would short circuit it and b) it tends
to melt if you turn up the power too high, and c) torches are really cheap and easy and fast.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Torches are a pain in the ass for maintaining temperatures that high I have tried it many times in something almost exactly like your suggesting and
it hardly manages to reach temperature. The outter surface gets plenty hot but it seems to have heat transfer issues thru the Iron pipe. It can be
done with a torch im sure but I can assure you its much more of a pain in the ass and produces a lower temperature then electric heating.
Im using a metal vessle, I scraped the ceramic tube I was going to use for this because the temperature rise is so fast it risk cracking and I like to
be able to crank that baby up and let it go, all thats needed to avoid shorting it out is a very thin layer of fiberglass wrapped around the tube. I
coat fiberglass in clay to make it harder and resist melting. If you have an issue with Nichrome melting its because your using to thin of nichrome or
you do not have a long enough run of it.
Im thinking of taking apart my old toaster oven and using the thick .25" elements that it contains. There are 4 of them and they will be much more
stable then wire Nichrome. This was bought from the Good Will for around $4 so its no big loss. These also come with a Voltage controlling noob and
connections with ceramic ends to make the entire process much simpler and more professional contruction in the end.
I have experimented enough now that im very confident that my next setup will yeild success. The only variable is going to be the Iron form that the
nichrome will be around. I normally use Copper tube which is much thinner but I think the only issue its going to cause using Iron pipe will be that
it will take slightly longer to heat up. Since it reaches the needed temperature with ease in around 10-15 minutes im not to concerned with a little
extra time added to the reaction.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
You definitely need the insulation; have you tried it with rockwool? That worked for me, although my yield wasn't that great, but my ratios were all
screwed up too so I figured that was why. Iron is conductive enough, the problem is you're losing heat faster than you can add it.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I use a furnace cement known as Cal-coat which is a mixture of Kaoline, fiber glass, and some calcium materials to act as a flux most of the time but
recently I found adding Kaoline to fiber glass to be much better at retaining heat and after the first firing it become rock hard yet porous enough
that less then a half an inch is all thats needed to heat one side and keep my hand on the other. Calcium in the cal-coat causes the mixture to melt
to low for my liking and around 2000 degrees F it starts to liquify.
I still would recomend anyone to try what im talking about with the Nichrome wire to see the difference, the heat is more direct and instant and it
quickly turns the iron or copper tubing into a white hot glowing mass and thats at only half power controlled with a light dimmer switch. This causes
all of the temperature issues normally associated with Phosphous production to go out the window and the contruction of the tube is simple enough that
I made the one earlier in under an hour.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
So do you use this CalCoat to put a thin layer over the iron, then wrap it with nichrome, then put a thick layer on after that? That was the problem
I typically had, was how to wrap a conductive metal object with nichrome wire.
Still, I don't remember needing the retort heated to more than an orange glow in order to generate phosphorus. Although I used ammonium phosphate
fertilizer, not bones like I said to use. I suppose that might make a difference.
Anyway, to help out, here's a nice chart correlating glow color with temperature: 
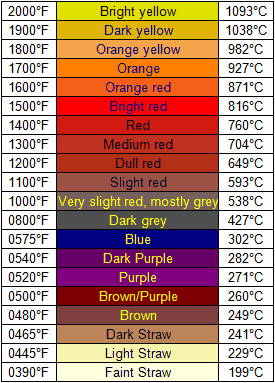
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
The thin layer is that pink insulation you find in you house dipped into a watery suspension of clay. A piece of insulation fluffed up to about 1.5"
will flatten down to a layer a little over 1/8 of an inch and when its dry and fired it turns into a rock. I then wrapped the old one in some round
Galvinized duct like what the heat in your house flows and packed the Cal-coat in it. This time I just used a mixture of Fiberglass, clay, and sand. I
know the temperature I reached is well over whats needed but I was just playing with it any way. I honestly would put the color of the thing between a
mix of the light straw and the Orange-Yellow. The reaction im performing is said to work at around 550C which would be around the slight Red range.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Perhaps I'm overlooking something obvious, but it seems the use of SiO<sub>2</sub> with the NaPO<sub>3</sub> reduction is
unnecessary.
3NaPO<sub>3</sub> + 5Al ----> 3NaAlO<sub>2</sub> + Al<sub>2</sub>O<sub>3</sub> + 3P
The fusing of Na<sub>2</sub>O with Al<sub>2</sub>O<sub>3</sub> and the fusing of Na<sub>2</sub>O with
SiO<sub>2</sub> are both similarly enthalpically favorable.
I weep at the sight of flaming acetic anhydride.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Meglar I got wounderful news, those new elements I spoke of are called Calrod and here is a picture of them,

The great thing about these is that they are already ceramic coated and non conductive on the outside. It is the same type of elements that used in
electric stoves.
I just made a quick run with the ones I ripped out of an old toaster oven and within 10-15 minutes the Pyrometer was at 2000F and the Copper tube
started to melt away so I stopped.
Amazing down right amazing.
Anyone that wants to make Phosphorus needs to use these, they will save you time and hassle no doubt.
@ Madscientist.
Heres the explination from the first page of this thread, the reaction without the SiO2 only produces roughly 30% yeilds where as with the Silica it
frees the remaining P and gives almost quantative yeilds.
Quote: Originally posted by Polverone  |
Aluminium as a Reducing Agent, &c.
L. Franck. Chem. Zeit. 1898, 22, [25], 236-245.
In:- The Journal Of The Society Of Chemical Industry. 17, [6], 612-613.
June 30,1898
Action of Aluminium on Phosphorus Compounds—Phosphorus vapour when led over powdered aluminium, heated to a dull red beat in a current of hydrogen,
combines with it with incandescence, forming a dark greyish-black unfused mass, which is decomposed in contact with moist (normal) air, forming PH3,
and leaving a greyish-white powder. It is decomposed also by water, aluminium also by water, aluminium hydroxide and a brownish-black residue being
left ; and by acids and alkalis, which dissolve it almost completely with evolution of PH3. The compound remains unaltered when heated in air.
At more or less elevated temperatures, all phosphoric, acid compounds (meta-, pyro-, and ortho-salts alike) are decomposed by aluminium.
Metaphosphates, however, undergo the most complete change, according to the equation—
6NaP03 + 15AI = 6NaAl02 + 2Al203 + Al5P3 + P3
The addition of silica effects the release of the remaining phosphorus, thus :—
6NaPO3 + 10AI + 3Si02 = 3Na2Si03 + 5Al203 + 3P2
Calcium and magnesium salts are as efficacious as those of sodium, but the superphosphates of commerce are not available for the production of
phosphorus in this manner. If, however, bone ash be decomposed by hydrochloric acid instead of by sulphuric acid, a material suitable for the purpose
is obtained.
Hence phosphorus may be produced, with almost quantitative completeness of yield, at relatively low temperatures...
|
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Ok so,
I didn't have much time to play this weekend since all that partying got in the way but I did learn a few things that will move this process
along to success in short order as long as I dedicate the time to it.
I tryed to use the setup I was going to which involved a piece of 2" Iron pipe with another slightly smaller one fashioned into a retort to place
inside of this setup. Theory was that it would act just like a simple kiln and fire that bad boy up to temperature in no time however reality proved
otherwise. Yes, the temperature was rising pretty well inside of the pipe but it was going very slow even with added insulation. I concluded after
observing it a few cycles that it could indeed reach the desired temperature and produce Phosphours however I have seen that these temperatures can be
reached much quickers and with a more stable pace using a thin walled material like what I did in my initial test using Copper tubing.
The solution was a simple one and it links back to one of the very few people in this extremely long over discussed thread where the fellow used an
old can of some sorts and a fire pit to produce the Phosphorus temperatures thru a simular reaction which I am about to undertake.
I made some rapid alterations and wrapped an old empty CRC electronic cleaning can in insulation and nichrome wire to see what it was able to
accomplish. I cut the top off so I could examine the internal reaction and from what I seen if one was able to fashion a simple replaceable, reusable
retort out of a container such as this using my sort of setup then the production of Phosphorus would be as simple if not extremely more so then the
process put forth by the person using a firepit and a retort who IIRC waited sometime, possible in the order of hours, for everything to reach
temperature. I observed my setup, which in the end will be almost the same as that persons, reach the same temperatures in a matter of minutes or less
instead of hours of waiting, watching, and stoaking a fire.
I promise you all simple Phosphorus without a doubt folks. I have felt for some time that this reaction has been given way more bad juju then need be
and it seemed that everyone would rather cry about high temperatures instead of just over comming them with simple materials. Your Getto master Sedit
promises he will make sure that if you follow his lead Phosphorus will be within the reach of even the dumbest of chemist, after all... if I manage
it.... its already been made by the dumbest of all chemist. .... Wish me luck. .... Wish me luck.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Good luck. You'll need it.
|
|
|
| Pages:
1
..
29
30
31
32
33
..
60 |