| Pages:
1
..
29
30
31
32
33
..
40 |
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i know this is not chemistry but i think its pretty too...and its still science!
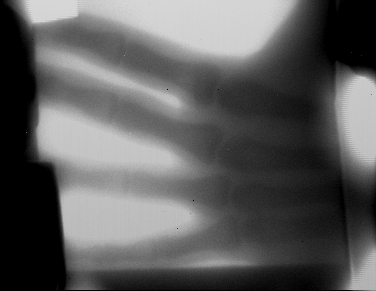
made an xray machine...this is my hand...
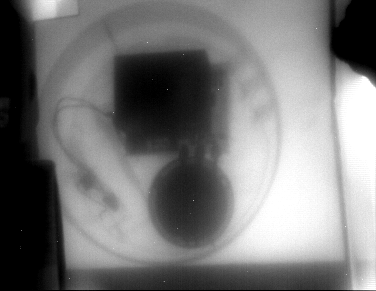
a smoke detector...
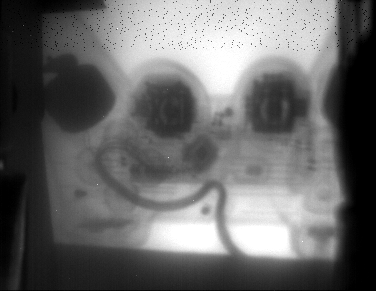
and a playstation2 controller..
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
We have just made some metallic silver from scrap, approx. 250g with a ~99,99% purity.
It was melted in a ceramic crucible with an oxygene/methane torch, under circa 1h. The silver was liquified under a layer of borax (it prevents the
oxidation of the molten silver). After all have melted we just dropped it in a bucket of water and took out this shiny lump of this beautiful metal.
[url=http://labphoto.tumblr.com]
 [/url] [/url]



I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
nice! solid chunk of Ag!
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Are those stray pixels from x-rays hitting your camera sensor? Yikes
I can't imagine what it was like for Rontgen to expose the first x-ray picture and see the bones showing.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
the camera was protected somewhat from too much radiations, and was aimed at a mirro reflecting the fluorescent screen ....
but yes those pixels are being fried you can tell the protective screen was not high enough in the last picture!
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Iodine crystals
Yet another one of my forgotten experiments sitting on my work bench. I was going to throw the plastic bottle containing the solution in the trash
until I saw some iodine crystals forming on the sides of the bottle. I plucked one out and did my best taking some macro shots with my cell phone
camera. I also photographed a couple more right through the plastic bottle.





That last one looks wicked - like something I'd put at the end of a spear. I hope to be able to store the crystals once harvested.
Tank
Chemical CURIOSITY KILLED THE CATalyst.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Not mind-blowingly pretty pictures, I thought they looked nice though 
1: Copper turnings reacting with acidified thiourea solution (see "low pH oxidation of thiourea" thread for details.
2: Nitration of thymol in a hot (80c) water bath.
 
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Nitrating thymol... sounds amazing! Could you PM me with the procedure?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
For sure! I followed the procedure from "Systematic Lab Experiments in Organic Chemistry" by Arun Sethi. The process is to dissolve 200mg thymol in
2ml 98% H2SO4. Once this is done add 4 ml of a nitrating mixture composed of 2ml conc HNO3 and 2ml conc H2SO4. Heat on a hot water bath for 15
minutes, filter, wash with cold distilled water, and recrystallize from dilute HCl if desired. I obtained about 215mg of the crude product.
Link to the book in question: http://books.google.ca/books/about/Systematic_Laboratory_Exp...
[Edited on 8-3-2013 by Mailinmypocket]
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I saw this post the other day on Reddit.
http://imgur.com/sIDB73G
http://www.reddit.com/r/pics/comments/19voto/this_rock_crack...
[Edited on 10-3-2013 by Morgan]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
What type of mineral could that be??
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I found a rock that kind of has the same strange melted look to it. It's not like a typical quartz or obsidian where the surface is glassy smooth, but
rather plastic looking almost with little bits of color if you turn it this way and that. It's a strange texture like that rock. It's kind of hard to
see in these photos but maybe you can make it out. Perhaps metal oxide interference is making the hints of color in my rock, very faint glints and
flecks, some inside the fissures.
  
|
|
|
Sublimatus
Hazard to Others
  
Posts: 108
Registered: 8-6-2011
Member Is Offline
Mood: No Mood
|
|
Maybe apatite (a calcium phosphate mineral)? Where did you find it?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I found just such a rock at a construction site when I was a child too. It looked almost like a broken piece of glass, but the crystal cleaved along a
horizonal plane.
[Edited on 10-3-2013 by AndersHoveland]
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Morgan, it looks like a piece of shattered tempered glass subjected to atmosphere for quite a long time. Hence the iridescence.
At first, when I saw the thumbnails, I thought it might fluorite, but upon closer look, it does look too glassy.
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I was thinking the waxy look of the surface is as if a piece of glass or quartz was flash heated to smoothen the surface. The glossy luster isn't like
the flat face of a crystal, but having slight ripples in places too.
It might something like this and because it's clear, I didn't recognize how obsidian kind of has those ripples in places. A few months ago I decided
to crack apart a large rock of black obsidian to see what kind of cutting tool would come about. A nice 10cm edge broke off that was very sharp. The
thinness of the glass where it was sharp was a lighter smokey color instead of a black opacity in bulk.
http://en.wikipedia.org/wiki/File:Conch_fract_glass.jpg
http://en.wikipedia.org/wiki/Conchoidal_fracture
"Several researchers have used scanning electron microscopy to determine the effect of heat treatment on cherts, a variety of microcrystalline
quartz."
"They found that heat-treated cherts fracture to form much smoother surfaces. In unheated cherts, a fracture goes around the grains and microcrystals,
leaving a rough surface. In heated cherts, the fracture goes through the microcrystals themselves, which results in a much smoother surface. This
smoother surface reflects light more evenly and produces an increased surface luster or "greasy" texture that is quite visible and serves as an
indication of heat treatment."
http://donsmaps.com/heatflint.html
http://donsmaps.com/images3/conchoidal.jpg
[Edited on 10-3-2013 by Morgan]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Needing 10g of NH4Cl I titrated 10% NH4OH with ~6M HCl to a methyl red endpoint (pH = 4). I then placed the solution in my family room window sill.
After a couple days crystals are now forming:

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have a 10...15 kV DC generator. This is the device:

When it is switched on, then the little wire at the left of the device shows a purple glow and makes a soft hissing noise. You also can feel a wind
coming from this wire. It is a corona discharge:

This is quite spectacular. There is only one end, the current simply flows through the air! The other end is at the other wire, shown in the picture
of the device.
I intend to use this device for high voltage experiments with gas discharge tubes.
[Edited on 11-3-13 by woelen]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | . . . makes a soft hissing noise. |
"Soft" is a four-letter word, woelen . . .
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
. . . and you're playing with (St. Elmo's) fire!
The small amounts of ozone produced are good for air-cleaning a smoky room, and such.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Is that really a DC generator? I've heard that only AC produces this one-wire-glow. I might be wrong.
Rest In Pieces!
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Quote: Originally posted by Magpie  | Needing 10g of NH4Cl I titrated 10% NH4OH with ~6M HCl to a methyl red endpoint (pH = 4). I then placed the solution in my family room window sill.
After a couple days crystals are now forming:
|
Ammonium chloride makes such nice snowflake crystals. I filmed a video of a saturated solution cooling, looked like a blizzard in a flask
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Quote: Originally posted by Adas  | | Is that really a DC generator? I've heard that only AC produces this one-wire-glow. I might be wrong. |
indeed you are! 10-15Kv AC is fun but will not generate the wind of charged particles mentioned above. only DC can do that.
I have a 40Kv for the Xray machine and a 100Kv for other stuff...
if you like to play with vacuum tubes and high voltage you should totally get you a Cockrof Watson ladder!
10-15 Kv is the lowest you can do anything with you've got to get more volts!!
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
A mind-blowing photograph of the eruptions of <a href="http://en.wikipedia.org/wiki/Sakurajima" target="_blank">Sakurajima Volcano,
Japan</a> <img src="../scipics/_wiki.png" />, February 2013, taken by <a href="http://www.mrietze.com/web13/japan13.htm"
target="_blank">Martin Rietze</a> <img src="../scipics/_ext.png" />:
<img src="http://www.mrietze.com/images/japan13/Jp13-084-5DIIdet.jpg" />
<iframe sandbox width="560" height="315" src="http://www.youtube.com/embed/XTns__G_F1U" frameborder="0" allowfullscreen></iframe>
(Note; the audio is re-synced to match the visual.)
[Edited on 7/9/13 by bfesser]
|
|
|
platedish29
Hazard to Self
 
Posts: 76
Registered: 2-9-2012
Member Is Offline
Mood: absorbing CO2
|
|
Brassy, gold rain. More of a "golden rain" because in fact there is no gold in there.


Ever heard of those drinks with parcicles of gold inside which worth a fortune? Now you can have it!Or tell your friends you do (at least).
(ammonium)? ferrous phosphate suspension.
(looks nasty, I've put too much a dosage)
Thanks rstar from whom the idea has been stolen! 
[Edited on 20-3-2013 by platedish29]
|
|
|
| Pages:
1
..
29
30
31
32
33
..
40 |