| Pages:
1
..
28
29
30
31
32
..
81 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by idrbur  | Somedays before i tried to make PA with aspirin but i obtained only few crystals .so i tried again but this time i doesn't get precipitate so i cold
the solution to -3 degree but it doesn't worked .so again performed the process with new batches 2-3 times but i doesn't get ppt. Any time .
Can anybody tell me how can i precipitate picric acid from the solution. |
Maybe you could use an organic solvant to extract picric acid from the mix...BTW What is in it?
Assuming you have only HNO3/H2SO4 and picric acid, you may allow the all batch to react with excess NH3 (NH4OH); then heat and evaporate (if needed)
then cool down to 0°C.
I have found some datas on solubilities of NH4 picrate, NH4NO3, NH4HSO4 and (NH4)2SO4 from various sources and by crossing the datas, I got a diagram.
Datas for NH4HSO4 in red have been extrapolated from phase diagram of NH4HSO4.
If you use excess NH3; there will only be (NH4)2SO4 in solution...
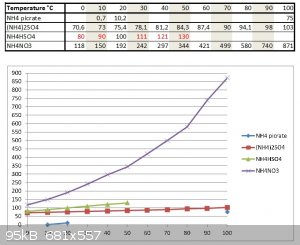
From that you see that NH4 picrate is the least soluble and at 0°C in excess NH4(+) media from more soluble NH4NO3 and (NH4)2SO4, it should be
virtually unsoluble (very close to 0,0 gr/ 100 gr water...)
So after filtration allow to react with 30% HCl and soluble NH4Cl will form while unsoluble picric acid will remain.
[Edited on 1-1-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
nitration process is one of the steps of synthesizing high explosive. I am thinking what EM that has no nitration at all. I figure out peroxide ,
permanganate ,chlorate and perchlorate families are the only ones.
peroxide family is very sensitive and has RE factor = 0.8 and VoD = 5300 m/s
permanganate /chlorate/perchlorate is less sensitive than peroxide but less powerful , VoD ~ 2500 m/s and max RE ~ 0.25
do we have other EM that has no nitration step and still not sensitive and has acceptable power?
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Other energetic materials that are not made by nitration would include:
* Azide explosives
* Picrate explosives
* Heavy metal acetylides
Be good, otherwise be good at it 
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
I guess technically picrates do involve nitration to make picric acid as the starting material but Mercury and silver fulminate, silver oxalate,R-salt
and Tetrazoles don't. RDX technically is not made via nitration either. PLX is another example along with FAE type mixtures. So I guess there's a
few.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Liptex
Quote: Originally posted by ecos  |
nitration process is one of the steps of synthesizing high explosive. I am thinking what EM that has no nitration at all. I figure out peroxide ,
permanganate ,chlorate and perchlorate families are the only ones.
peroxide family is very sensitive and has RE factor = 0.8 and VoD = 5300 m/s
permanganate /chlorate/perchlorate is less sensitive than peroxide but less powerful , VoD ~ 2500 m/s and max RE ~ 0.25
do we have other EM that has no nitration step and still not sensitive and has acceptable power? |
Well, it is maybe question on me. This plastic explosive exist and it is Liptex. His power is see here:
https://www.youtube.com/watch?v=gyx2f9b2HFg
VoD is about 6000 m/s at density 1,5 g/cm3. This is density in video. At density 1,85 g/ cm3 is VoD 7000 m/s. Produce:
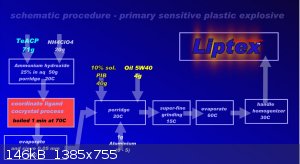
Process without nitration, acids. Time stability in closed container (15C) without limits. Free on air by 15 Celsia about 30 days, without explosive
changes. This EM Slowly spend molecule NH3, evaporate. Minimum kick from 300mg LA. Or his equivalent. Standard kick No.8. From density 1,8+ is
possible used as EM for EFP, SC etc. Oxygen balance has near nil. "C" in scheme is Celsia temperature during process.
Dr. Liptakov
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Nice LL, is that your own invention?
Does anyone know the difference between lead azide that has been coated with dextrin and lead azide that has been coated with PVA during synthesis?
Would one be safer or are they essentially the same in sensitivity?
Be good, otherwise be good at it 
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Litpex
Well, it is not from any patents. Inventor is only Dr. Liptakov, special madness on field of energetic materials research.
(unfortunately, about Lead azide I know nothing)
Doc. :-)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by ecos  |
nitration process is one of the steps of synthesizing high explosive. I am thinking what EM that has no nitration at all. I figure out peroxide ,
permanganate ,chlorate and perchlorate families are the only ones.
peroxide family is very sensitive and has RE factor = 0.8 and VoD = 5300 m/s
permanganate /chlorate/perchlorate is less sensitive than peroxide but less powerful , VoD ~ 2500 m/s and max RE ~ 0.25
do we have other EM that has no nitration step and still not sensitive and has acceptable power? |
There are a lot of other explosive materials than nitration involved ones. See explosophoric groups or hazardous chemicals and lab chemical hazard
reactions compendium to get a good general picture.
The assumption that permanganate, chlorate, perchlorate are less powerful than peroxides is wrong, you are probably refering to the ammonium salts...
Except maybe for permanganate, hydrazinium, guanidinium, methylaminium, hydroxylamonium, ethandiaminium, ... nearly all amines salts of oxoanions are
much more powerful than peroxydes (VOD >6500 m/s).
Some may even be close or better than RDX or HMX.
Nitroaromatics may be accessed without HNO3 but from nitrite.
ex: Cl-CH(-CH=O)2 + AgNO2 -aceton-> O2N-CH(-CH=O)2 + AgCl
O2N-CH(-CH=O)2 (nitromalonaldehyd) -trimerisation/decarboxylation-> 1,3,5-Trinitrobenzen
Aromatic iodoxy compounds are explosive. Ar-IO2
Aromatic diazonium salts are explosive. Ar-N=N(+) especially with oxoanions (nitrate, perchlorate, ...)
Aromatic perchloryl compounds are explosive. Ar-ClO3
Liquid oxygen explosives.
N2O4 explosives (panclastites).
Bromate amine salts.
Iodate and periodate amine salts.
...
Very long list of reactions and examples.
[Edited on 1-2-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Greenlight,
From, "Proceedings of the Symposium on Lead and Copper Azides", GW Taylor, 1966:
"It is possible to suppress spontaneous explosion by the addition of small amounts of various additives such as dextrin, polyvinyl alcohol and other
hydrophilic colloids.
It is suggested that spontaneous explosion may be associated with nucleating conditions and not with super-sensitivity due to the presence of internal
strain and sudden release of stresses in large crystals."
Dextrin is almost always listed first from what I have seen, but it appears that they both do more or less the same thing. Not completely sure though.
The following was taken from, "Technology of the Inorganic Azides, Energetic Materials Vol. 2":
"
A breakthrough came with the development about 1930 of dextrinated lead azide, which was considerably safer to handle than the unmodified material but
still powerful enough to function satisfactorily in contemporary detonators and related devices. Dextrinated lead azide was the first initiating
explosive to be manufactured* under carefully controlled conditions so as to produce an explosive with a more desirable range of properties.
Subsequent developments followed the same principles and employed crystal-modification agents, or "phlegmatizers" (e.g., dextrin, polyvinyl alcohol,
etc.), to produce a balance between reliable initiation, output energy, and safety in handling. Products were successfully developed to be free
flowing for ease of introduction into small diameter detonators and to have a high bulk density to provide the maximum energy in pressed compacts. The
trend in detonator and explosive-train design, which has continued into the 1970s, has been to smaller components, requiring decreased amounts and
diameters of more efficient explosives. This trend itself has tended to emphasize the technological importance first of lead and then silver azide and
to assure the continued modification of their properties by process development and control. In the United Kingdom the prewar development of Service
lead azide represented a trade-off of some handling convenience and safety for increased output. During World War II, polyvinyl alcohol (PVA) lead
azide was developed in the United States and resembled Service lead azide in appearance, output, and other properties. Then at the end of World War
II, work began on lead azide modified by precipitation in the presence of sodium carboxymethylcellulose (CMC) [1]. Originally, CMC-type lead azide was
developed to have the self-binding properties of dextrinated lead azide, so that it could be pressed into sleeves, yet have the initiation and output
properties of the Service material. Later development in the United Kingdom led to a variety of new explosive products given "RD" serial numbers and
in particular to RD1333 and RD1343 CMC-type azides. American versions of RD1333 were produced in both government plants and private industry and were
ultimately produced in large batches as Special Purpose lead azide. CMC-type lead azide had supplanted to a high degree but not completely replaced
Service lead azide, PVA lead azide, or dextrinated lead azide for military use in the United States, the United Kingdom, and most countries of the
North Atlantic Treaty and South East Asia Treaty Organizations. Dextrinated lead azide remains the principal product for civilian use, for mining and
demolition applications in general, and some military items. Other azides have been considered for use as detonants, but besides lead azide only
silver azide possesses a combination of properties which has found favor in industry. However, in spite of its long-recognized virtues [2], no process
was developed that would make silver azide in a form suitable for pouring and pressing into detonators. During the early 1950s, Taylor [3] and
Williams and Peyton [4] developed processes for making granular silver azide. Taylor's process [3] was adopted for use in British ordnance factories,
but it yielded only small (1.5 kg) batches in the standard British production vessels, and four hours were required to complete a batch. "
Given the preference given by the military to PVA lead azide, maybe it is superior in some ways to dextrinated lead azide.
Here are a couple references for you (I have just skimmed them a bit so far):
Attachment: Proceedings of the Symposium on Lead and Copper Azides - GW Taylor 1966.pdf (5.2MB)
This file has been downloaded 2226 times
Attachment: Technology of the Inorganic Azides - Energetic Materials Vol 2.pdf (8.4MB)
This file has been downloaded 2967 times
[Edited on 1-2-2016 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Thankyou for that information Hennig, as I am trying to decide whether to use dextrinated or PVA lead azide for the initiating charge in my detonators
and it is a great help.
In the second file (Technology of the Inorganic Azides) it says this about PVA lead azide:
"In the United States it is manufactured solely by the Olin Matheson Corporation using patented pro- cedures [9] which give little insight into the
actual manufacturing techniques used or the problems encountered. PVA lead azide is 96% pure and may contain some polyvinyl alcohol combined with
lead, but not in the same manner as the lead dextrinate in dextrinated lead azide, for the product consists of transparent, well-defined crystals. It
has an initiating efficiency equal to Service lead azide and, according to data published in the patent, about the same handling and sensitivity
properties."
It seems that there is more information available about the properties of the dextrinated lead azide than the PVA variant.
It also states that the dextrinated lead azide has a purity of 92%, maybe it takes up extra phlegmatizer during synthesis.
Be good, otherwise be good at it 
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Quote: Originally posted by PHILOU Zrealone  |
There are a lot of other explosive materials than nitration involved ones. See explosophoric groups or hazardous chemicals and lab chemical hazard
reactions compendium to get a good general picture.
The assumption that permanganate, chlorate, perchlorate are less powerful than peroxides is wrong, you are probably refering to the ammonium salts...
Except maybe for permanganate, hydrazinium, guanidinium, methylaminium, hydroxylamonium, ethandiaminium, ... nearly all amines salts of oxoanions are
much more powerful than peroxydes (VOD >6500 m/s).
Some may even be close or better than RDX or HMX.
Nitroaromatics may be accessed without HNO3 but from nitrite.
ex: Cl-CH(-CH=O)2 + AgNO2 -aceton-> O2N-CH(-CH=O)2 + AgCl
O2N-CH(-CH=O)2 (nitromalonaldehyd) -trimerisation/decarboxylation-> 1,3,5-Trinitrobenzen
Aromatic iodoxy compounds are explosive. Ar-IO2
Aromatic diazonium salts are explosive. Ar-N=N(+) especially with oxoanions (nitrate, perchlorate, ...)
Aromatic perchloryl compounds are explosive. Ar-ClO3
Liquid oxygen explosives.
N2O4 explosives (panclastites).
Bromate amine salts.
Iodate and periodate amine salts.
...
Very long list of reactions and examples.
[Edited on 1-2-2016 by PHILOU Zrealone] |
Liquid oxygen explosives. --> those are peroxides which are very sensitive !
I think azides are used as primary explosives because they are quite sensitive.
N2O4 ---> I couldn't find much info about explosives having this compound
I will explore the rest.
[Edited on 4-2-2016 by ecos]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by ecos  | Quote: Originally posted by PHILOU Zrealone  |
There are a lot of other explosive materials than nitration involved ones. See explosophoric groups or hazardous chemicals and lab chemical hazard
reactions compendium to get a good general picture.
The assumption that permanganate, chlorate, perchlorate are less powerful than peroxides is wrong, you are probably refering to the ammonium salts...
Except maybe for permanganate, hydrazinium, guanidinium, methylaminium, hydroxylamonium, ethandiaminium, ... nearly all amines salts of oxoanions are
much more powerful than peroxydes (VOD >6500 m/s).
Some may even be close or better than RDX or HMX.
Nitroaromatics may be accessed without HNO3 but from nitrite.
ex: Cl-CH(-CH=O)2 + AgNO2 -aceton-> O2N-CH(-CH=O)2 + AgCl
O2N-CH(-CH=O)2 (nitromalonaldehyd) -trimerisation/decarboxylation-> 1,3,5-Trinitrobenzen
Aromatic iodoxy compounds are explosive. Ar-IO2
Aromatic diazonium salts are explosive. Ar-N=N(+) especially with oxoanions (nitrate, perchlorate, ...)
Aromatic perchloryl compounds are explosive. Ar-ClO3
Liquid oxygen explosives.
N2O4 explosives (panclastites).
Bromate amine salts.
Iodate and periodate amine salts.
...
Very long list of reactions and examples.
[Edited on 1-2-2016 by PHILOU Zrealone] |
Liquid oxygen explosives. --> those are peroxides which are very sensitive !
I think azides are used as primary explosives because they are quite sensitive.
N2O4 ---> I couldn't find much info about explosives having this compound
I will explore the rest.
[Edited on 4-2-2016 by ecos] |
Liquid oxygen explosives are not peroxydes...peroxydes means that the molecule contains the linkage R-O-O-R' where R, R' may be H, Alkyl, Aryl, or
Acyl like:
CH3-O-O-CH3 (methyl ether peroxyde)
CH3-O-O-H (peroxymethanol)
H-O-O-H (water peroxyde)
C6H5-CO-O-O-H (perbenzoic acid)
CH3-CO-O-O-CO-CH3 (peracetic anhydride)
LOX is simply a mix of liquefied O2 and a porous fuel; It simply burns fiercely when set in flame in the open, maybe it may D2D when confined, and it
detonates from detonator.
Some organic azides are not as sensitive as the mineral ones...
CH3-N3
N3-CH2-CH2-N3
N3-C6H2(NO2)3
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Many of the compounds in the recent family of energetic salts do not involve nitration at all (eg. aminotetrazole nitrate), and some employ borderline
techniques such as dehydrating a nitrate to form a nitro compound (eg. aminonitroguanidine and its nitrate salt). Many of these are at the absolute
cutting edge, performance-wise.
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Quote: Originally posted by Microtek  | | Many of the compounds in the recent family of energetic salts do not involve nitration at all (eg. aminotetrazole nitrate), and some employ borderline
techniques such as dehydrating a nitrate to form a nitro compound (eg. aminonitroguanidine and its nitrate salt). Many of these are at the absolute
cutting edge, performance-wise. |
a compound that has a nitrate in its name would have a nitric salt or nitric acid treatment !!!
It is very hard now to me to access AN or diluted nitric acid thats why I want to avoid any nitration process
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Ecos, If you haven't ammonium nitrate and nothing nitric acid, will be construction any EM pretty difficult. Maybe will be better put question, what
you have. After will be answer easy. Doc.
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
He is right, not having the ability to nitrate compounds take a massive chunk out of the energetics you will be able to use.
Can you get potassium nitrate as fertilizer where you are and sulfuric acid. If so you can distill your own nitric acid.
Be good, otherwise be good at it 
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
As you might know, orgsyn.org publishes procedures tha usually provide some range of yields or maybe it's two numbers (one is ht eyield of original
researcher, one is the yield of checkers). I was not able to find any explanation of the reason two numbers are usually provided for yields. Does
anyone know?
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ecos  | Quote: Originally posted by Microtek  | | Many of the compounds in the recent family of energetic salts do not involve nitration at all (eg. aminotetrazole nitrate), and some employ borderline
techniques such as dehydrating a nitrate to form a nitro compound (eg. aminonitroguanidine and its nitrate salt). Many of these are at the absolute
cutting edge, performance-wise. |
a compound that has a nitrate in its name would have a nitric salt or nitric acid treatment !!!
It is very hard now to me to access AN or diluted nitric acid thats why I want to avoid any nitration process |
Nitration is not the same as incorporating a nitrate anion, eg. by metathesis. If you can't buy simple precursors such as nitric acid or nitrate
salts, then preparing these should be your focus. Everything is made from chemicals, it's "just" a matter of refining it into the chemicals you need.
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Thanks a lot for your replies.
I have access to them but with small quantity. I have tons of questions when i buy AN grade fertilizer or diluted nitric acid (65%).
I just want to get rid of this headache.
I am thinking now to synthesis nitric acid , I am just reading now about how to do so through oxidation of ammonia gas.
I will write posts about this soon.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I always wanted to try bacterial fermentation like was done with the old nitre beds, but using more modern feed, equipment and techniques. For
starters instead of manure and urine I would use fertilizer grade urea, and I would use a substrate able to soak up solution and still allow good
aeration (peat moss?). Forced air from a blower would be used to provide proper air flow through the ferment. The temperature of the bed would be
maintained at an optimum level with an electrical heater (heat the inlet air?). Even moisture level could be controlled; a moisture sensor could be
used to monitor moisture level and the associated controller could spray the substrate/ferment as needed. IIRC, I have read that nitrates can be
produced by fermentation in a fraction of the time as was achieved with the old fashioned nitre beds using modern techniques such as these. I have at
least one or two journal articles discussing this, but they are on a different computer in another city.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Yes, you could see such a deficiency in reactants as a golden opportunity to develop not only your chemical skills, but also your skills in chemical
engineering (whether you went with bacterial nitrogen fixation or a more classical high voltage method or catalytic oxidation of ammonia). There are
lots of interesting options.
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
OB%
Just a quick question regarding oxygen balance.
My last batch of PE was made just using PETN as the energetic. I know that a perfect oxygen balance improves the explosives detonation properties.
PETN is slightly underoxidized (-10%) and ETN is overoxidized slightly @ 5.2-5.3%.
Would a plastic explosive from a ratio of PETN to ETN that has been calculated to 0% be more powerful than the straight PETN plastic explosive or
would the binder and plasticizer throw out the balance during detonation.
[Edited on 10-2-2016 by greenlight]
Be good, otherwise be good at it 
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Plastic explosives are normally used in applications where maximum brisance and the ability to "disrupt" the target are of main importance. So
extremely high brisance explosives, high energy density, with components mixed at the molecular level are typically what is desired. I don't think the
oxygen balance of something not mixed at the molecular level is normally of great importance here, especially an inert plasticizer, binder mixture.
I thought this was kind of interesting.
Retrieved the following from the archive "Yarchive":
"
From: glhurst@onr.com (Gerald L. Hurst)
Newsgroups: alt.engr.explosives
Subject: Re: What is C4 made of?
Date: 11 Mar 1997 22:22:04 GMT
In article
, Daniel
John Krut says:
>I am a chemistry student at the U of C and am wondering how C4 is produced
C-4 is a simple mechanical mixture of powdered RDX (cyclonite) and
a plasticizing mixture in the ratio 91:9. The specific plasticizer
mix is comprised of:
Polyisobutylene 2.1
Motor oil 1.6
Di(2-ethylhexyl) sebacate 5.3
There is no particular magic in the specific plasticizer mix other
than that it has good stability and low-temperature plasticity. It
may be viewed as an inert diluent as far as explosive properties go.
Jerry (Ico)
"
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
OB
Counting oxygen balance for molecular explosive give nonsense. Thus, brisance ETN, PETN, RDX (and his mix) give highest brisance with minimum
plasticizer (and other compounds, color etc). For this compounds OB does not count. Example: RDX plast with 9% PLF super brisance
PETN with 9% PLF good brisance.
ETN with 9% PLF less brisance.
Overall "power" is giving of content main of explosive (or most powerfull + main) of explosive in %. Different condition is for AN Fx. Here is OB from
zero to - 10 very important. However, still applies rule, than overall "power" give main explosive compound. For example, A N + urea will be weakly,
than AN + Hexamine. Problematic about it is of course more complex issues. This explanation is simplified for easy understanding. ETN + PETN on zero
OB + PLF 9% will be weakly than PETN + PLF 9 %.
Doc.
[Edited on 10-2-2016 by Laboratory of Liptakov]
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
What is PLF ?
|
|
|
| Pages:
1
..
28
29
30
31
32
..
81 |