| Pages:
1
2
3
4
5 |
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
And, how are you going to deal with that nice chlorinated decomposition product COCl2, commonly called phosgene? One member is believed to have died
from phosgene poisonings a few years back. Do you even have a citation to prove what you said? I seriously doubt that this will give oxalyl chloride
in anything above dismal yields. You should also give more information regarding what you mean by chlorinated products, just saying that they are
derived from ethylene oxalate does not tell us anything.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Triflic Acid, I found some patents while ago, right before getting flamed by Eldritch. Patents are arguably not te best source of information but
there is atleast some literature to support this dubious proposition.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Quote: Originally posted by Triflic Acid  | | And, how are you going to deal with that nice chlorinated decomposition product COCl2, commonly called phosgene? One member is believed to have died
from phosgene poisonings a few years back. |
Go outside and wear a gasmask. Am I missing something?
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Oh, when he said decomposition, I immediately thought that he was doing something around pyrolysis. A hot bubbling phosgene gyser is not the best
thing to deal with. If he's doing it with a tertairy amine its safer.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
@mack indeed you are missing something, namely the fact that a supplied air respirator is necessary according to the CDC (but that's just the man trying to keep us amateur chemists down). So a surplus Israeli gas mask that has a 40 year old filter might not cut
it. And the rules say no phosgene. And no source is provided.
Right now I can't imagine a decomposition of "chlorinated ethylene carbonate" that doesn't involve carbonate itself, which would form water in its own
decomposition thus destroying some product. But I could be totally off.
Reflux condenser?? I barely know her!
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Probably if you work with it all the time under the fan you would need the supplied air respirator.
But to be honest, when you're outside and in your gasmask, you could even hold the breath when you get close to it to make sure you don't expose
yourself too much.
Of course any accident would be really dangerous, but it's probably not that bad
|
|
|
MaeBorowski
Harmless

Posts: 12
Registered: 8-3-2021
Location: six feet under
Member Is Offline
|
|
There is an option to dump it on the absorption column, but it would be better to use it to produce new ethylene carbonate or put it in some solvent
like toluene or other useful purposes for the chemist. This is quite dangerous, however, I have some experience with highly toxic gases (in my case
carbon monoxide, but phosgene is much, much angrier) outside the fume hood. Don't do this yourself.
It can be concluded that the product of the exhaustive chlorination of ethylene oxalate will be tetrachloroethylene oxalate 
I apologize for not mentioning the release of phosgene in the ethylene carbonate method. I tried to find a rational method through one's lens and
completely forgot about the rules. Thank you for pointing out my mistake.
To be honest, I have no sources for the direct reaction of oxalic acid with ethylene glycol. In my opinion, there are no theoretical obstacles in this
synthesis: water is distilled with carbon tetrachloride, which shifts the equilibrium, and oxalic acid is strong enough to carry out the reaction
without a catalyst. It should be remembered that the difference between theory and practice is much greater in practice than in theory 
please leave comments about my English in PM
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | It can be concluded that the product of the exhaustive chlorination of ethylene oxalate will be tetrachloroethylene oxalate  |
This is a pretty stark example of textbook chemistry with no consideration of the real world. Free-radical chlorinations like you refer to run slowly
at low concentration and low temperature with a strong UV light. Running that can be pretty annoying.
Could it work? Yeah. Is it practical? Probably not. Could it fail? Yes: HCl byproduct might cleave the ester before chlorination completes. Is it
basically the same thing as free-radical chlorination of glyoxal, which was suggested almost immediately? Yes. Does it satisfy the conditions of the
bet? No: phosgene production is pretty likely when Cl* radicals interact with oxalate...
EDIT: third idea: rxn of 1,2-dibromoethane with two equivalents of phenol under Ullmann condensation conditions to 1,2-diphenoxyethylene, then Br2,
DBU, NaNH2, 2xCl2. Same as the previous with a different way of forming the vinylene-1,2-diether.
[Edited on 28-3-2021 by clearly_not_atara]
|
|
|
MaeBorowski
Harmless

Posts: 12
Registered: 8-3-2021
Location: six feet under
Member Is Offline
|
|
You're goddamn right. But only partially: the product of chlorination isn't a tetrachloroethylene oxalate. In reality, chlorination requires 6.76 mol
of chlorine versus the theoretical 4 (carbonate 4.93 mol vs the same 4), which indicates that there are changes in the oxalic acid residue. It is
strange that in the production of oxalyl chloride, phosgene is released from tetrachloroethylene bis-chloroformate, which, in theory, should be
obtained by breaking the carbon-carbon bond in the oxalic acid residue, but phosgene isn't obtained from the "tetrachloroethylene oxalate"
itself, according to the data. Now I am even more interested in this idea.
The synthesis took place under a reflux. UV, I think, can be replaced by direct sunlight. This was used in some older manuals.
By hydrolysis?  Most esters are perfectly resistant to hydrogen chloride. Most esters are perfectly resistant to hydrogen chloride.
Something in me says that we will get hydrogen chloride and carbon monoxide instead of the coveted oxalyl chloride.
In the process of decomposition — possibly, in the process of chlorination — excluded. If there is phosgene, it will be in such concentrations
that hydrogen chloride is a bigger problem.
Quote: Originally posted by clearly_not_atara  | | third idea: rxn of 1,2-dibromoethane with two equivalents of phenol under Ullmann condensation conditions to 1,2-diphenoxyethylene, then Br2, DBU,
NaNH2, 2xCl2. Same as the previous with a different way of forming the vinylene-1,2-diether. |
In Russian, to describe such syntheses, there is a stable expression "to hammer nails with a microscope". This is a possible way, I agree, but
everyone who can repeat it has a) a fume hood b) several kgs of oxalyl chloride 
[Edited on 29-3-2021 by MaeBorowski]
please leave comments about my English in PM
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Okay, your chemistry isn't wrong, you just don't seem to understand what JJay wanted. As to why, I don't know, he left the forum in a huff some months
ago. Phosgene simply isn't allowed. It might be contained by someone sufficiently dedicated, but its generation is strongly discouraged here due to
the sneaky lethality and common use of improvised safety equipment, which are a bad combination.
That kinetically stable chloroformates appear, rather than phosgene being released, does make sense to me. That is an interesting process.
When I said "free radical chlorination of glyoxal", I undersold the problems with this route, I agree. Homolytic cleavage of the radical intermediate
is an issue I didn't consider. I'm not convinced it will happen, but polymerization is another problem.
How about free-radical chlorination of phenoxyacetyl chloride? Now that's three steps:
phenol + chloroacetic acid >> phenoxyacetic acid + SOCl2 >> phenoxyacetyl chloride etc.
EDIT: free-radical chlorination is not required, ordinary enol chlorination will do
[Edited on 29-3-2021 by clearly_not_atara]
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
Is there a way from oxalyl bromide to oxalyl chloride? Or is it only possible from chloride to bromide? Bromine is much easier to handle than chlorine
I think
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
I just saw something on prepchem: https://prepchem.com/oxalyl-chloride/. It is the ethyl carbonate synth, but sure enough, it makes phosgene. Also, I think that it is only a one
way thing going from bromide to chlorine, https://www.prepchem.com/synthesis-oxalyl-bromide/.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
MaeBorowski
Harmless

Posts: 12
Registered: 8-3-2021
Location: six feet under
Member Is Offline
|
|
I've worked with both chlorine and bromine. The second one is much more smelly and corrosive, and, moreover, not cheap.
The problem with bromine is the need to dry it. This requires additional synthesis steps, whereas to produce dry chlorine, you only need a chlorine
generator and a flask of concentrated sulfuric acid. Due to the gaseous state, you can not worry about contamination and assemble the glasswares on
paraffinized rubber plugs.
please leave comments about my English in PM
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Has someone already mentioned the benzyl alcohol to benzoyl chloride reaction? Then Benzoyl chloride + oxalic acid -> Benzoic acid + oxalyl
chloride reaction. This seems like the most OTC reaction to me. The oxalyl chloride boils out pushing the equilibrium to the right-hand side
[Edited on 22-5-2021 by Opylation]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
BzCl + H2C2O4 >> BzOH + CO2 + CO + HCl
Next!
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Some benzoic anhydride seems likely, as it's a general method for anhydrides. Same for benzotrichloride...I wonder what the yield of benzaldehyde from
benzal chloride and oxalic acid is.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Ouch! hahah oh well, I guess using BzCl for acyl chloride preparation can't be used for everything. There is a neat Thionyl Chloride preparation from,
I think, phthalic anhydride and SO2. Let me see if I can find it
EDIT: Curiously, would using alkyl oxalate esters stabilize the reaction?
EDIT2: Found it! I also had a screen grab of a post from this forum, but didn't include the poster. I apologize in advance for not giving credit to
that person but they pointed me to this paper. It actually uses phthalyl chloride which reacts to form phthalic anhydride in a reversible reaction
EDIT3: I also found another paper regarding the use of tetrachloroethylene carbonate as a precursor, which can be prepared from ethylene carbonate and
chlorine. The issue with this method is that it also produces phosgene, which isn't the nicest of chemicals to work with. Since it's produced in-situ
however, maybe using a double wash bottle; the first you're preferred absolute carboxylic acid (double product fun!), and the second being either
sodium hydroxide solution or ammonium hydroxide solution to scrub the remaining phosgene. I'm sure if you use concentrated sulfuric acid as grease and
perform this entirely outside with the proper precautions it could be done responsibly.
EDIT4: I'm sorry, I guess I'm beating the dead horse on that last mention. That method has been mentioned plenty in this thread
Attachment: kyrides1937.pdf (318kB)
This file has been downloaded 609 times
Attachment: Process for the Production of Oxalyl Chloride (US Patent 2816140) (1).pdf (293kB)
This file has been downloaded 560 times
[Edited on 23-5-2021 by Opylation]
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
I was just thinking back to the ethylene carbonate -> chlorination -> oxalyl chloride + phosgene reaction and got to thinking would the
transesterification of dialkyl oxalate to ethylene oxalate work? This forms 1,4-dioxane-2,3-dione. Theres a similar compound, Dioxalin, that is formed
with oxalic acid and glycerol but the branching carbon group may stabilize it. I couldn't find any information on the formation of the 2,3-dione of
dioxane which leads me to believe it's too unstable. But, if it were possible to form this compound, than the tetrachlorination followed by heating
would liberate 2 moles of oxalyl chloride?
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
That's a really brilliant idea. Happily, 1,4-dioxane-2,3-dione is known, CAS# 3524-70-7. The link shows that it is normally made from ethylene glycol and ... oxalyl chloride.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
I found a paper on cyclic oxalate ester formation and properties. It looks like ethylene oxalate has a high propensity to hydrolyze and
polymerize/demolymerize. However, the paper does mention a preparation, so if used immediately it might be possible to prepare the tetrachloro
derivative.
P.S. it uses transesterification for the prep 
Attachment: carothers1930.pdf (2.5MB)
This file has been downloaded 548 times
[Edited on 4-6-2021 by Opylation]
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Just would like to provide an update that I have found a patent on the preparation of tetrachloroethylene oxalate via the transesterification of
dialkyl oxalate and ethylene glycol followed by UV radical chlorination in carbon tetrachloride. Seems like a decent procedure to bump/update the
thread for anyone who is curious 
Attachment: Tetrachloroethylene oxalate.pdf (435kB)
This file has been downloaded 527 times
[Edited on 8-6-2021 by Opylation]
|
|
|
BauArf56
Hazard to Self
 
Posts: 68
Registered: 22-8-2019
Location: between the moon and the sun
Member Is Offline
Mood: energetic
|
|
if this reaction is reversible, first oxalic acid could be treated with bromine (which can be made from pool sanitizer sodium bromide), getting oxalyl
bromide. Then it could be treated with hydrogen chloride gas, yielding oxalyl chloride.
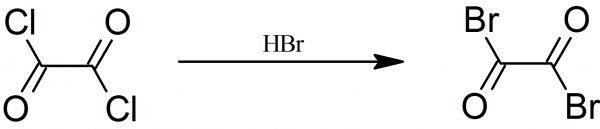
[Edited on 8-6-2021 by BauArf56]

|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Liquid bromine and Oxalic acid react to form hypobromic acid, carbon monoxide and carbon dioxide. Hypobromic acid reacts with oxalic acid to form
hydrogen bromide, water, and CO2
|
|
|
BauArf56
Hazard to Self
 
Posts: 68
Registered: 22-8-2019
Location: between the moon and the sun
Member Is Offline
Mood: energetic
|
|
forgot that bromine is an oxidiser! How about using hydrogen bromide instead of bromine? HBr could be made in situ with sulfuric acid and sodium
bromide
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
This thread doesn't need more random guesses from people who didn't bother to study the problem at all. HBr will not brominate any carboxylic acid,
much less oxalic.
|
|
|
| Pages:
1
2
3
4
5 |