| Pages:
1
2
3
4
5
..
25 |
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
I can't remember if I've mentioned this before (and this connection is too slow for me to bother looking), but I have a fair amount of
5-aminotetrazole. I'd be happy to sell/trade some to anyone who's interested...
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
How are you going to be able to legally send such an highly explosive compound through the post? BTW How did you acquire or make the stuff?
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
It seems fairly benign to me. If you heat it on a spoon for example it will decompose slightly energetically, but I certainly wouldn`t call it
explosive. The nitrate salt is pretty cool, if you powder it (probably not very safe!) and soak a bit of NG into it then it`s impressive, to say the
least... As for how I acquired it, well, that`s a secret  . .
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
lol I remember from an old post on roguesci how you got it...you trickster you .
1kg yes? .
1kg yes?
Aminotetrazole is non explosive, it decomposes non-explosevly at around 205 C IIRC.
I have a hundred or so grams of aminoguanidine, so I am happy, I doubt I will ever need more for the small scale experiments I do.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
Wow, I`d almost forgotten about roguesci! I remember that when I got the stuff I was wondering if I could do a decyclisation to get an azide. But then
I got myself some of that, too. Scamming became almost an addiction at one point! 
(2kg  ) )
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by The_Davster
A previous limitation in the prep of nitrotetrazoles from 5-ATZ was the nature of the acid copper nitrotetrazolate salt, Cu(NT)2HNT,(NT=CN5O2-)
This patent here: http://www.freshpat ents.com/Primary-explosives-dt20060209ptan20060030715.php mentions:
"Ammonium nitrotetrazolate was prepared by diazotization of 5-aminotetrazole in the presence of excess nitrite followed by extraction as the
tri-laurylamine salt and displacement by ammonia. Upon addition of stoichiometric amount of ammonium hydroxide, sodium nitrotetrazolate forms
quantitatively and is analytically pure."
I have seen nothing in the literature on such a synthesis, I wonder if it is some sort of in-house method at LANL or something?
|
I've made NH4NTZ solution in such method: dissolve Cu salt with excess of Ba(OH)2 in water, boil until CuO settles down, filter it off and measure
weight to calculate amount of nitrotetrazole ion in solution. After this add solution of (NH4)2SO4 (1 mole for 1 mole initial Ba(OH)2), filter
unsoluble BaSO4 and you have NH4 salt solution with some dissolved ammonia, whitch is removed by boiling. Although i'm not isolated NH4 salt i found
that it is very soluble. Also i found that condidions on whitch NH4NTZ and Fe salts are mixed, are important. After addition of FeCl2 and 2h boiling i
got no precepitate. But then i dissolved CoSO4 or CuSO4 in sample taken from solution, i got pink or blue precipitate of corresponding
nitrotetrazolate. On addition of K2CO3 to sample and slight heat i got ammonia smell. So i concluded that solution contains NH4 nitrotetrazolate, so
my reagents are ok. Some special conditions are needed to form NH4FeNT salt. I'm sure in that. Also i found that in patent they use Fe(ClO4)2 instead
of FeCl2 used in green primaries article. Perchlorate anion very unlikely goes to inner sphere of complex compound, so this change may have some
reason, may be to assist complex formation or to minimize side reactions, but i am not sure.
[Edited on 11-9-2007 by Engager]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
I have succeded making 5-nitotetrazole - ammonium complex NH4CuNT. Compound formula (NH4)2[Cu[NTZ]4(H2O)2]. I've made it by following method: Solution
of 5.5g ammonium 5-nitrotetrazolate in 38 ml of water added with stiring to solution of 2.52г Cu(NO3)2*6H2O in 110 ml H2O. A small quantity of
blue precipitate is formed emidately. Solution was heated on boiling waterbath for 4 hours, solution becames transparent blue. It's slowly cooled to
room temperature and after to 10C in freezer. Large quantity of blue "snowy" precipitate is formed, solid is filtered off, washed with ice cold water
and with small portion of ice cold alcohol. Product was air dried. Photo of product shown below:
According to patent data, density is 1.94 g/cm3, detonation velocity 7390 m/sec (at 1.71 g/cm3). Insensitive to spark up to 0.36 J (human activity
generates up to 0.25 J), sentive to shock 23 cm (vs 9.6 PbN3 and 14 PETN), slightly sensitive to friction 0.6 kg (vs 0.01 PbN3 and 5.8 for PETN).
Thermicaly stable up to 265C, detonation products volume is about 750 l/kg, products of explosion: N2, CO2, H2O, ~2% NO2, ~3%CO. Oxygen ballance (CO)
is zero. Substance is stable on air, light and moisture. Almost completely safe then wet, even with open flame. In dry state flame contact takes DDT
(deflagration-detonation transition).
I've also tried Fe and Co complexes. Attempt with FeCl2*6H2O was unsuccesfull, as i've mentioned in previous post. Attempt with Co(ClO4)2*6H2O gave
success but yield was low. I guess there are some special conditions that need to be satisfied then making Fe and Co complex 5-nitrotetrazolates.
[Edited on 26-8-2007 by Engager]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by Rosco Bodine
See PATR , Vol.1 , A-569
The sodium salt is produced in 76% yield from a solution of
5-aminotetrazole in 15% NaOH at 50C by oxidation with KMnO4 solution added dropwise . Unreacted KMnO4 is
decomposed with alcohol , and the solution is refluxed for 1 hour at 100C . On cooling the sodium salt is obtained as
crystals . |
Synthesis of Na-5,5'-Azotetrazolate
Method was tested with success. 5-Aminotetrazolate monohydrate (10 g) was dissolved with stirring in 40 ml of 15% aqueous NaOH solution at 50°C
(dissolves amlost permanently, solution is colorless). Another solution of 10 g of KMnO4 in 50 ml of hot distilled water was prepared. The aqueous
KMnO4 solution was then added slowly into the stirred aqueous NaOH solution of 5-aminotetrazolate monohydrate (small gas evolution and heating were
observed). Resulting solution is dark green with some brown precipitate. Into this mixture 10 ml of ethanol was added to react with excess KMnO4,
sollution color is turned to brown/black. Then, the reaction solution was refluxed at 100° C. for 1 h. The resulting reaction mixture was then
filtered. Upon cooling, yellow crystals of sodium 5,5'-azotetralate dihydrate (SZT) crystallized from the filtrate gradually. The crude product was
recrystallized and dried to give 9.13 g (76.4%) of pure SZT. Photo of product crystallizing under mother liquer, and solid product on filter shown
here:
Reaction scheme:
[Edited on 11-9-2007 by Engager]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
I've also made two other tetrazole-based energetic compounds. Diazoaminotetrazole and dihydrazinium 5,5'-azotetrazolate (mentioned as substance with
highest known positive heat of formation).
Synthesis of sodium bis-5,5'-diazoamintotetrazolate from aminoguanidine bicarbonate
Prepare mixture of 11.5 ml 70% nitric acid with 100 ml of water, add by portions with stirring 24.8g aminoguanidine bicarbonate. Stir mixture until
CO2 evolution stops and then add 20.4 ml 70% acetic acid. Mixture is stirred and slightly heated until all solid dissolves. The resulting clear
yellow solution is solution of aminoguanidine nitrate in 12-13% acetic acid. This solution is cooled in freezer to 3-4C, well mixed and placed on ice
bath. Slowly, with stirring, by small portions at time ice cold solution of 17.5g sodium nitrite in 75 ml of water is added. While addition,
temperature must be all times kept below 12C, perfectly in interval of 5-7C, process takes about 30-40 minutes. After diazotation is finished mixture
is removed from ice bath and left to stand at room temperature for 24 hours. Some time after removal from ice bath slow evolution of nitrogen begings,
and mixture can heat up to 25C, this is ok, so don't worry, and after about 12-16 hours of standing evolution of nitrogen stops and large amount of
diazoaminotetrazole precipitates. Solid is filtered off, washed with ice cold water slightly acidified with acetic acid and left to dry at room
temperature. Yield is about 50% of pure mono-sodium salt of diazoaminotetrazole. Photos of product shown below:
Reaction scheme:
[Edited on 11-9-2007 by Engager]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Ooh very nice , this one is certainly worth pursuing. Azotetrazolate is a dianion , its salts can
have two cations or ligands. It too would be interesting to see if a polymer resin can be formed
with formaldehyde.
| Quote: | Dihydrazinium 5,5’-Azotetrazolate
Synthesis in water produces yellow needles of the dihydrazinium salt [N2H5]2:[N4C-N=N-CN4].
Heat of formation is + 1147 kcal kg one of the highest ever reported. The compound is stable at
room temperature, almost insensitive to friction and impact, but detonates violently when the
explosion is initiated, e.g., by rapid heating over the decomposition temperature or by using an
initiator.
5,5’-azotetrazolate salts show the remarkable insensitivity to electrostatic discharge, friction
and impact while having a very high heat of formation.
|
Dihydrazinium 5,5’-Azotetrazolate Dihydrazinate Complex

http://pubs.acs.org/cgi-bin/abstract.cgi/inocaj/2001/40/i14/...
US patent 5877300
Preparation of guanidinium 5'5-azotetrazolate
Variations on tetrazolates _
Ionic Liquids as Energetic Materials
http://stinet.dtic.mil/cgi-bin/GetTRDoc?AD=ADA464308&Loc...
.
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
I've already made dihydrazinium azotetrazolate. I can post metod of synthesis with photos if somebody interested. It's yellow needle like solid,
soluble in water.
[Edited on 11-9-2007 by Engager]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Synthesis of dihydrazinium 5,5'-Azotetrazolate (HZT)
Prepare solution of 4.8g sodium 5,5'-azotetrazolate in 30 ml boiling water and 5.58g barium chloride dihydrate in 15 ml of boiling water. Solutions
are mixed and stirred, precipitate of barium 5,5'-azotetrazolate forms emidately, solution is cooled to 10C and filtered. Ba salt is washed with small
amount of ice cold water and dried at room temperature. Yield is about 6.2g.
Make solution of 5.3g hydrazine sulphate (N2H6SO4) in 155 ml of water, 6.44g of barium hydroxide is added with stirring, and after 6.2g of barium
5,5'-azotetrazolate is added. Mixture is well stirred for 1 hour, solid (BaSO4) is filtered off and discarded, resulting in yellow solution of
dihyrazinium 5,5'-azotetrazolate. Solution of HZT is placed on boiling water bath and heated until most of water evaporate and first crystals of HZT
form. Solution is then removed from water bath and cooled to room temperature and after in freezer to 10C. Mixture almost comepletely solidifies to
form yellow needles of dihydrazinium 5,5'-azotetrazolate dihydrate. Crystalls are filtered of and dried at room temperature. Yield is about 87%.
Anhydrous salt may be obtained by drying dihydrate in vacuum exicator at 100C for 2 days.
I've already made dihydrazinium azotetrazolate. I can post metod of synthesis with photos if somebody interested. It's yellow needle like solid,
soluble in water. Photos shown below, left photo is barium azotetrazolate, two others are dihydrazinium azotetrazolete (HZT):
  
Reaction scheme:
[Edited on 14-9-2007 by Engager]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by Engager
Synthesis of sodium bis-5,5'-diazoamintotetrazolate from aminoguanidine bicarbonate
|
What is the referance for this procedure? I have not came across it, and I have read much of the azotetrazolate literature.
| Quote: | Originally posted by franklyn
It too would be interesting to see if a polymer resin can be formed
with formaldehyde.
|
If the chemistry is analogous to that of nitrotetrazole, an alcohol will be formed, in the case of nitrotetrazole NO2CN4CH2OH is formed
[Edited on 10-9-2007 by The_Davster]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Synthesis of 5-Aminotetrazole (ATZ)
Thiele method. 34g aminoguanidine-bicarbonate is dissolved in 217 ml of 15% nitric acid (36ml of
70% HNO3 + 185 ml water). Mixture is stirred until CO2 evolution stops and all solid dissolved. Resulting solution is diazotized by solution of 17.2g
sodium nitrite in 35 ml of water. Nitrite solution is added slowly with stirring, while reaction mixture is cooled on ice bath, temperature must all
times kept below 20-25C. Diazotation is proceeding smothly with neglible evolution of NOx, if mixture is foaming (result of HNO2 decomposition),
addition must be paused and mixture must be well stirred to stop foaming, before new portions of nitrite added. Addition takes time about 10-15
minutes. After addition of nitrite is completed, mixture is allowed to sit for 20 mins at room temperature, and 29g of Na2CO3 (or 46g of baking soda)
is added by portions with mixing. Mixture is stirred untill CO2 evolution stops and all excess of bicarbonate fully dissolves. Mixture is placed to
round bottom flask with attached reflux condenser and boiled for 4 hours. Resulting solution of 5-aminotetrazole is acidified by 30% H2SO4 to pH=4,
and left to cool to room temperature. Usualy crystallization of product starts around 40C, but solution have great tendency to supersaturate. If after
cooling to room temperature crystallization is not started, seed crystall of aminotetrazole is introduced (made by placing glass rod with drop of
solution to alcohol), or inner side of flask (below solution of course) is rubbed with glass rod with intense friction. After crystallization is
started solution is left at room temperature for 12 hours, and cooled to 10C in freezez. Crystalls of 5-aminotetrazole are filtered, slightly washed
with ice cold water and dried at room temperature. Yield is 13.6g (64%). Photo of product will be shown at bottom of post.
Reaction scheme:

Schtolle method. Warning!!! This method includes work with
exteremely dangerous, explosive and highly toxic hydrogen azide. Concenration of it's solution must be kept below 20% all the times because of severe
explosion hazard (<20% are explosion safe). All work must be done with good ventilation, and fumes must not be inhaled in any circumstances (HN3 is
very volatile, and is effective protoplasmic poison, causes blood cells decomposition and severe headaches). Never add sodium azide to cold acids
solutions - it may result in condensation of drops rel. conc. HN3 on cold walls of flask, and can explode with extreme violence. Method of synthesis
is optimized for maximum safety, but precautions must be remembered all times. Dissolve 10.5g of dicyandiamide and 16.25g of sodium azide in 250 ml of
water heated to 50C. Mixture is stirred untill all solid dissolve, and 2.15 ml of 36% HCl is added dropwise with stirring, mixture is left at room
temperature for 12 hours, then, after heating to 50C 4.3 ml of 36% HCl is added in same way, mixture is left for 6 hours, next 6.45 ml and 2-3 hours,
and finaly remaining 8.6 ml of 36% HCl. After addition is completed mixture is left standing at room temperature for 1 week, white crystalls of
5-aminotetrazole must apper, but solution again may be supersaturated and no crystalls are precipitated - in this case crystallization must be started
as written in Thiele method above. Yield depends of standing time and puriry of reagents, i have ~50% (10.5g) yield after 2 weeks of standing, but
literature sources show yeilds up to 97% after longer standing. Pure snow white crystalls of 5-aminotetrazole are filtered off, washed with ice cold
water and dried at room temperature.
Reaction scheme:

Photos of products are shown below. Left photo is ATZ made by Thiele's method, and right one is photo of ATZ made by
Schtolle's method:
 
[Edited on 11-9-2007 by Engager]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by The_Davster
What is the referance for this procedure? I have not came across it, and I have read much of the azotetrazolate literature. |
Reference is russian book: Бубнов П.Ф.
Инициирующие
взрывчатые вещества и
средства инициирования
(часть 1). М., 1940. [P.F.Bubnov "Primary explosives and prime devices(part 1)" Moscow, 1940.] page 316
Original method uses aminoguanidine nitrate as starting material, i have modified it to generate it in situ from aminoguanidine bicarbonate. Method
was tested by me with success, photo is evidence if you don't believe. Also i want to post reaction mechanism so no further explanation will be
requiered:
[Edited on 11-9-2007 by Engager]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Somebody interested in synthesis procedures for BNCP [Tetraamino-cis-bis(5-nitro-2H-tetrazolato-N2) cobalt (III) perchlorate] and NH4CuNT
[5-nitro-1H-tetrazolato-N2 cuprate (II)], both newest edge priming explosive materials?
[Edited on 11-9-2007 by Engager]
|
|
|
artem
Hazard to Self
 
Posts: 53
Registered: 9-1-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | [quote/]
..dihydrazinium salt [N2H5]2:[N4C-N=N-CN4]. Heat of formation is + 1147 kcal kg one of the highest ever reported... |
Calculated value H=+1147kcal/kg (1105kJ/mole) is doubtful. Different estimations give no more than +715...750KJ/mole (743...779kcal/kg).
For example,
condensed polyacetylenes 2055kcal/kg(C4H2), 1570(C4N2)
azides 1467(HN3), ~1490(C(N3)4), ~1400 for C2N4(N3)2, N3CN4H, 1100-1200(C3N3(N3)3).
tetrazole 817
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It's nice to see interest in experimentation with these tetrazoles  Nice
crystals Nice
crystals 
Some thoughts ...
There are a few related things which
may be worth looking into , which were mentioned on the preceding page .
There are possible shortcuts to aminoguanidine from reaction of any guanidine salt with hydrazine via hydrazine sulfate . See US5041661 . And also
possibly dicyandiamide reaction with hydrazine sulfate may produce aminoguanidine . See US3285958 .
Guanylazide styphnate , and nitroguanylazide styphnate could also be very interesting 
As styphnic acid is a di-acid , the neutral salts would actually be (di)guanylazide styphnate , and likewise for the (di)nitroguanylazide styphnate
salt . There probably would be a basic lead salt for the mono-guanylazide styphnate and also for mono-nitroguanylazide styphnate .
Also the synthesis of aminotetrazole might produce higher yield via tetracene intermediate .
[Edited on 14-9-2007 by Rosco Bodine]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Synthesis of acid copper 5-Nitrotetrazolate (CuNT) from 5-aminotetrazole
Prepare solution 20.8g sodium nitrite and 11g of copper sulphate (CuSO4*5H2O) in 60 ml of hot water, resulting
solution contains copper nitrite and has dark green color. Prepare solution of 10.3g aminotetrazole (ATZ) and 0.4g CuSO4*5H2O in 12.8 ml 70% nitric
acid + 120 ml of water. Diazotetrazole is intermediate in nitrotetrazole synthesis, it can explode in solution if concentration will reach 2% from the
slightless stimulus, even at 0C. Microexplosions are not dangerous but acting on nerves. To completely avoid them, slow addition, effective stiring
and carefull temperature control are essential. Small adition of copper sulphate to ATZ solution is essential to avoid microexplosions in drops, on
contact with nitrogen oxides, escaping from reaction mixture. Copper nitrite solution is placed on ice bath and cooled to 5C, then solution of ATZ in
nitric acid, is added slowly with stirring (perfectly drop by drop). During addition temperature of reaction mixture must be kept below 15C all times.
If mixture begins to foam, addition is paused and mixture is well stirred until foam dissapears before next portions of ATZ solution are added
(foaming is result of HNO2 decomposition to water and NOx if it's concentration is too high). Reaction is proceeding smoothly, without
microexplosions, with small evolution of NOx, if conditions are carefully controlled. Whole addition process takes time about 1.5 hours. Close to end
of addition mixture becomes thicker, and changes color to green- blue (similar with homemade CuCO3*Cu(OH)2). After addition is completed mixture is
left to sit in ice bath for 15 minutes, after that 14 ml of 70% nitric acid + 6 ml of water is added with stirring. Reaction mixture is left for 1
hour, solid precipitate of acid copper salt of nitrotetrazole is filtered off, washed with 5.72 ml HNO3 + 44 ml H2O and with three portions of 50 ml
H2O. Yield is about 85%. Product is bluish - green crystals, almost insoluble in cold water.
Warning!!! Acid copper salt of nitrotetrazole is powerfull and sensitive explosive.
It is almost completely safe then wet, but in dry state it can explode violently on friction, impact or heating. Safety precautions must be
remembered all times.
Below are the photos of process. Left photo shows solutions of copper nitrite (left one) and ATZ in nitric acid with
CuSO4 added (right one). Photo in the middle shows ice bath with sitting reaction flask, and termocouple temperature control. Right photo shows
reaction product on filter.
Reaction sheme:
Acid copper salt of nitrotetrazole can be easily converted to soluble salts of nitrotetrazole by heating in solution
of corresponding hydroxides. For example solution of sodium 5-nitrotetrazolate can be prepared by boiling acid copper salt in NaOH solution:
Cu(NT)2*HNT + 3NaOH => 3NaNT + CuO+ 2H2O. Solid black copper oxide is removed by filtering, pure solution of NaNT can be concentrated to separate
solid salt, or can be used dirrectly for further reactions.
[Edited on 14-9-2007 by Engager]
|
|
|
Axt
National Hazard
   
Posts: 858
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Excellent posts Engager, but just a suggestion, dont use imageshack or other free hosts as inevitably within a month or two they will all end up dead
links. Instead either attach them directly of to imbed then in the text use the <a
href="http://www.sciencemadness.org/talk/viewthread.php?tid=603">forum pic ftp</a> setup specifically for this purpose. What program did you
use to draw the reaction schematics?
I recently prepared 5-nitraminotetrazole. Its properties given in Chem. Mater. 2007, 19, 1731-1739 are quite remarkable, its density via gas
pycnometry is 2.06g/cm which gives a calculated VOD of 10358m/s using the "Cheetah 4.0" program of LLNL. JACS, 73(5), 2327-2329 mentions that is a
sensitive explosive, though this is disputed by J. Org. Chem., 18(8), 941-945, saying that it wont explode when struck "very sharply" on an anvil with
a hammer. Though they dont say if they were testing the sensitivity of the hydrated or anhydrous acid. Thats another problem with nitraminotetrazole,
it retains solvent of crystalisation and water of hydration though this is said to be lost of standing at room temperature.
I prepared nitroaminoguanidine by condensing <a href="http://www.sciencemadness.org/talk/viewthread.php?tid=8911">nitroguanidine</a> with
hydrazine hydrate via the method of JACS, 73, 474, this is said by the authors to give a purer product in higher yield then the method of JACS, 50,
2465-2470 that used hydrazine sulphate-ammonia mixture of for the condensation. Below is the change in colour of the solution and the appearance of
nitroaminoguanidine crystals under the microscope.
<center><img src="http://www.sciencemadness.org/scipics/axt/nitroaminoguanidine-crystals.jpg"></center>
To see if this was indeed nitroaminoguanidine, the hydrazone derivative was produced by condensation with formaldehyde. On standing very long fine
needles separated which meets the description in JACS, 50, 2465-2470. The precipitate resembled fiberglass or nitrocellulose and when ignited
deflagrated vigourously with a large orange flame as shown below.
<center><img src="http://www.sciencemadness.org/scipics/axt/nitroaminoguanidine-formaldehyde-deflag.jpg"></center>
The nitrosation of nitroaminoguanidine was done following the experimental method "A" given in JACS, 73, 2327-2329, which uses a heated neutral
solution of nitroaminoguanidine and potassium nitrite, though modified it to use sodium nitrite. Cooling the solution resulted in precipitation of the
sodium nitraminotetrazolate but this was not isolated, rather HCl was added then it was extracted with ether, evaporation yielded ~5.5g of crude
5-nitraminotetrazole from 8g nitroaminoguanidine. Doing this probably resulted in some contamination with nitroguanylazide and its this contamination
that J. Org. Chem., 18(8), 941-945 speculates to be the reason form an increased sensitivity. This crude product on drying for a number of days at
room temperature exploded easily under the hammer on a rusty anvil and deflagrated in a flash on ignition as shown in the picture below.
<center><img src="http://www.sciencemadness.org/scipics/axt/nitraminotetrazole-deflag.jpg"></center>
A 1:1 molar aqueous mixture of AgNO3 and nitraminotetrazole were combined which resulted in an immediate white precipitate of the acid silver salt.
When dried this salt only "snappled" when held in a flame spreading the pile around but wouldnt sustain in the small quantities used.The literature
does mention that salts of nitroaminotetrazole are not initiating explosives with the possible exception of the mercury salt. The potassium salt was
also prepared by combining 1:2 molar ratio of nitraminotetrazole and potassium hydroxide in ethanol, again a white precipitate formed immediately
which was filtered and dried. This only deflagrated on ignition as shown below.
<center><img src="http://www.sciencemadness.org/scipics/axt/potassium-nitraminotetrazole.jpg"></center>
[Edited on 1-10-2007 by Axt]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by Axt
Excellent posts Engager, but just a suggestion, dont use imageshack or other free hosts as inevitably within a month or two they will all end up dead
links. Instead either attach them directly of to imbed then in the text use the <a
href="http://www.sciencemadness.org/talk/viewthread.php?tid=603">forum pic ftp</a> setup specifically for this purpose. What program did you
use to draw the reaction schematics?
[Edited on 28-9-2007 by Axt] |
ChemDraw Ultra 8.0 from ChemOffice 2004 Suite. Thanks for the advice, next time i will attach images dirrectly to posts. Now, getting back to topic.
I've recently made silver salt of 5-nitrotetrazole, and conducted some tests on AgNTZ senivity. Here is a small detail about this interesting
explosive material:
Silver nitrotetrazolate is white crystaline powder, stable to light and moisture, prepared by mixing soluble nitrotetrazole salt solution with silver
nitrate. Compound is powerfull primary explosive, heat of explosion 1.94 MJ/kg, initiating power is 0.005g vs tetryl (HgNTZ: 0.006g, Lead azide:
0.02g, Mercury Fulminate 0.2g). Silver 5-nitroterazolate is sensitive to shock and friction - slighly more then mercury fulminate.
Can be overpressed.
Then wet it can detonate on contact with flame, but is much less sensitive to friction and shock, then in dry state. I've made some friction and tests
on dry salt. On intensive friction between paper and wooden rood, no explosion was encountered. However attempt of grinding in mortar, resulted in
almost emidate violent explosion, with bright white flash. Sensivity to shock is very high, even slightest hits can cause violent detonation. In my
personal oppinion, silver nitrotetrazolate can be handeled in safe manner with little risk when threated right, but extreme caution is advised.
I've attached photo of silver 5-nitrotetrazolate, among other tetrazole derivatives i've made so far. Upper-left to lower-right: 1. Copper-ammonium
complex 5-nitrotetrazolate (NH4)2[Cu(N4C-NO2)4(H2O)2] - green primary (NH4CuNT) ; 2. (N2H4)2(N4C-N=N-CN4) - dihydrazinium 5,5'-azotetrazolate (HZT) ;
3. Cu3(N4C-N=N-NH-CN4)2 - copper 5,5' diazoaminotetrazolate (CuDAT) ; 4. Cu(N4C-NO2)2*(HN4C-NO2) - acid copper salt of 5-nitrotetrazole (CuNT) ; 5.
Ag(N4C-NO2) - silver 5-nitrotetrazolate (AgNT) ; 6. Na3(N4C-N=N-NH-CN4) - sodium 5,5'-diazoaminotetrazolate (NaDAT) ; 7. Na2(N4C-N=N-CN4) - sodium
5,5'-azotetrazolate (NaAT) ; 8. HN4C-NH2*H2O - 5-aminotetrazole monohydrate (ATZ).
[Edited on 1-10-2007 by Engager]

|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Since I was feeling left out in my pet thread...
I have been too busy to experiment!
But when I have time, time for these guys, among other tetrazoles.
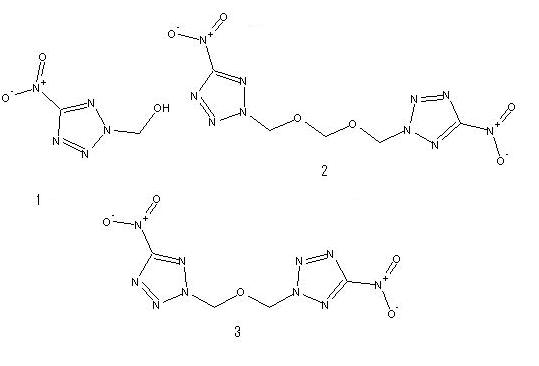
1) 2-hydroxylmethyl-5-nitrotetrazole
6.85g of sodium nitrotetrazole (hydrate) was dissolved in 50mL of water and 24.5mL of 20% sulfuric acid and 10mL of a 37% aqueous solution of
formaldehyde were added with stirring at 5-10 C. The mixture was kept for 24h at 18-20C and extraced with ether(3x50mL) and the extract dried over
magnesium sulfate and evaporated under reduced pressure to obtain 3.2g(67%) of compound 1 as a colorless crystalline substance, mp 63-64C
2) 2.6g of (1) was dissolved in 8mL of 101% sulfuric acid at 18-20C and 0.27g of paraform was added. The mixture was stirred for 1h at 35-40C, cooled
to 5C, and poured into 50g of finely crushed ice. The precipitate was filtered off and washed with 50mL of ice water. Yield 4g(80%), colorless
crystalline substance, mp157-158C
3)1.45g of (1) was dissolved in 20 mL of 101% sulfuric acid at 18-20C and the mixture was stirred for 2h at that temperature and poured into 100g of
finely crushed ice. The precipitate was filtered off and washed with 50mL of ice water. Yield 0.5g(35%), colourless crystalline substance, mp
165-167C
[Edited on 30-9-2007 by The_Davster]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
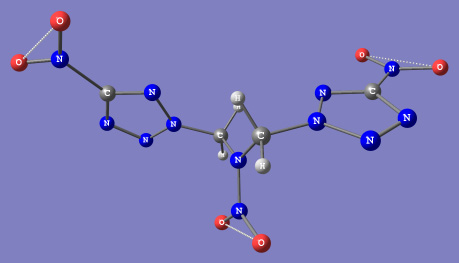
I've found a very interesting nitrotetrazole based compound in my russian explosive related documents, called HT-1:
1,3-di(nitrotetrazolato-N2)-2-nitro-2-azopropane. Structual formula computed by RHF is attached below this message. HT-1 can be made by reaction of
1,3-dichloro-2-nitro-2-azopropane with silver nitrotetrazolate in organic solvent:
Cl-CH2-N(NO2)-CH2-Cl + 2Ag(N4C-NO2) => (N4C-NO2)-CH2-N(NO2)-CH2-(N4C-NO2) + 2AgCl (precipitate)
Some properties of HT-1. First time prepared in USSR in 1972, melting point 165C, density 1,90 g/cm3, heat of formation +476 ccal/kg (according
different source 388 ccal/kg), heat of explosion 1430 ccal/kg, detonation velocity (density): 9350 m/sec (1.86), 9940 m/sec (1.90), critical diameter
0.82 mm. Thermal stability is acceptable.
I have fair ammount of Ag nitrotetrazolate, all that i need is to synhesis of 1,3-dichloro-2-nitro-2-azopropane. I had an idea to make this stuff
through the nitramide, whitch i've made by hydrolisis of N,N'-dinitrourea in water (watch dinitrourea thread). Wonder if this will work:
NH2-NO2 + 2CH2Cl2 => (ClCH2)2N-NO2 + 2HCl
Other ideas:
2CH2Cl2 + NH2SO3K (potassium sulfamate) => (ClCH2)2NSO3K + 2HCl => (ClCH2)2NSO3K + HNO3 => (ClCH2)2N-NO2 + KHSO4
(CH3)2NCOH(DMF) + HNO3 => (CH3)2N-NO2 + CO + H2O => (CH3)2N-NO2 + Cl2 => (ClCH2)2N-NO2 + 2HCl
Someone have any ideas about how 1,3-dichloro-2-nitro-2-azopropane can be made?
[Edited on 8-10-2007 by Engager]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
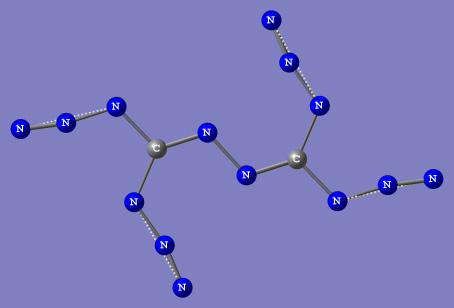
There is also an interesting route from 5,5'-azotetrazole (which is made by simple oxidation of aminotetrazole by KMnO4; watch my post somethere
above) to isocyanogen tetrazide (watch US patent 2,990,412 for method details). Compound can be easily made from isocyanogen tetrabromide and solution
of NaN3 in acetone/water. Trick is that isocyanogen tetrobromide can be simply made with good yield by action of bromine (in form of bromine water) on
solution of sodium azotetrazolate, nitrogen is evolved and product separates as black oily layer:
Na(N4C)-N=N-(CN4)Na + 3Br2 => 2NaBr + 4N2 + Br2=C=N-N-C=Br2
Action of sodium azide in acetone/water gives isocyanogen tetrazide with excelent yield:
Br2=C=N-N-C=Br2 + 4NaN3 => (N3)2C=N-N-C(N3)2 + 4NaBr
Not much digital data about explosive properties of compound exists, but it is certainly powerfull explosive. Structual formula calculated by RHF is
attached below this post. May be someone want to try this? This stuff is 100% real and excelenly described in original work of Thiele and in US
pattent i've mentioned. Unfortunately i can't try this myself due to the lack of good place for such experementation. Someone interested in trying
this stuff?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Engager, your posts in this thread are always a pleasure!
HT-1 is very similar to another energetic:

The preparation of which is in the attachment.
I know I came across the synthesis of 1,3-dichloro-2-nitro-2-azapropane while looking for the synthesis of 1-chloro-2-nitro-2-azapropane, I will use
scifinder at Uni on tuesday to look it up again.
Attachment: 1nitrotetrazolato2nitro2azapropane.pdf (113kB)
This file has been downloaded 2608 times
|
|
|
| Pages:
1
2
3
4
5
..
25 |
|