| Pages:
1
2
3
4 |
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Squirrel is correct. Phosgene oxime is not prepared, as I assumed, from hydroxylamine and phosgene.
It is prepared by reduction of trichloronitrosomethane with H2S or aluminum amalgam.
Or, from silver or mercury fulminate,
I never looked it up before, because it is a violent vesicant and I had no interest in its preparation, and still don't.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
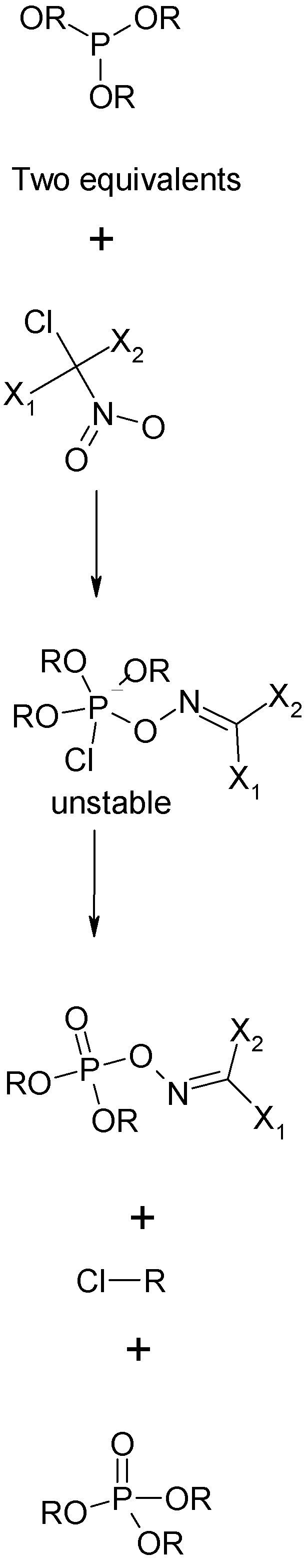
The novichok reaction is the "Allen Reaction" between two moles of a trialkyl posphite and one molea alpha-trihalonitroalkane, the most familiar such
compound being trichloronitromethane, (chloropicrin) Cl3CNO2. The much less familiar nitroso compound Cl3CNO also works. There are some constraints on
the choice of substituents.
1. One of these must be chlorine, this is what attacks phosphorus initially.
2. The others may be Cl, Br, F or a psuedohalogen e.g. -CN. These are the substituents that remain in the oxime ester side chain. These may be
identical or different.
The product is an exime ester of a dialkyl, dialkoxy, alkyl halo, or alkoxyhalophosphate.
See graphical scheme upthread and below.
The preparation of the chloropicrin and analogs is well known.
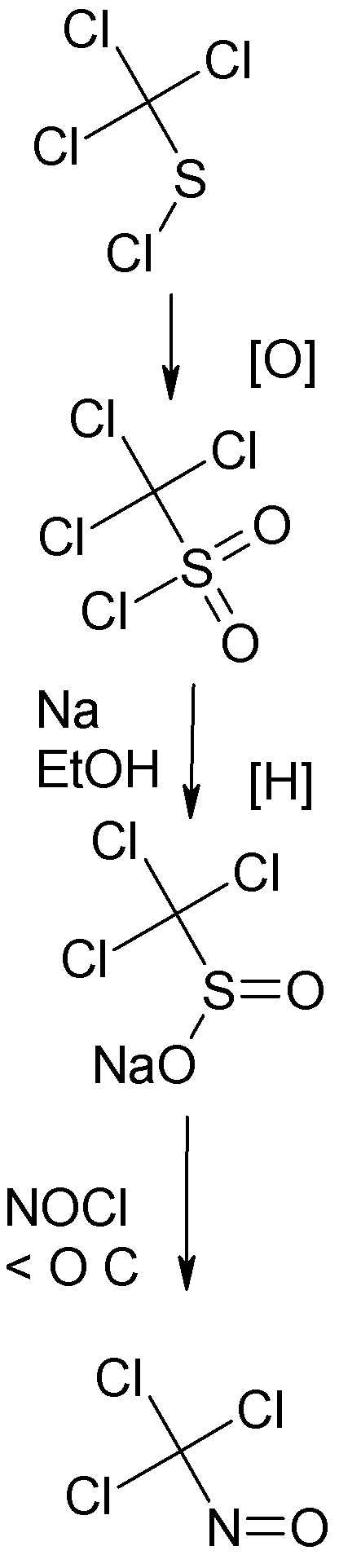
The most convenient prep of trichloronitrosomethane is one starting from trichloromethyl sulfenyl chloride, also known as perchloromethyl mercaptan,
the familiar product of chlorination of CS2 with dry chlorine in diffuse light.
This is oxidized to trichloromethylsulfonyl chloride according to the procedure of Prandl and coworker in Ber., 62 1752, (1929). A technical grade of
this may be available from Eastman Kodak.
The sulfonyl chloride is reduced with sodium in ethanol by the technique of Prandle and coworker in Ber. 65 (1932). The product is sodium
trichloromethylsulffinate.
The procedure described in same peper for preparing the trichloronitrosomethane, a deep blue liquid, from the sodium salt of the
trichloromethylsulfinic acid is simple but causes some thermal decomposition and also produces a product not free from water.
The same salt can be reacted in the cold with nitrosyl chloride in liquid phase, under autogenous pressure, the reaction being complete when the
mixture is allowed to warm to O C. This procedure is detailed in a paper by Suttcliffe in JACS 79 3071 (1975). The anhydrous
trichloromethylnitrosomethane is well suited for use directly in the Allen reaction.
Allen made no reference to any special precautions being necessary for the preparation of the simple oxime esters he characterized.
I would not assume them to be nontoxic, however.
It is clear now that the basic chemical principles behind the novichoks have been known to the United States for at least thirty three years. It is
worth noting that Allen was a chemist employed by FMC Corporation and that corporation was a major contractor involved in the VX program.
At least three reported novichok structures have been described in detail, those being A-240, A-242, and A-244. Are these compounds AChE inhibitors
possessing toxicities of military potential? It would be simple enough for someone in an appropriate facility to find out. But they aren't talking,
one way or the other. Neither confirmation nor denial.
The questions are hanging there.
[Edited on 5-7-2008 by Sauron]
[Edited on 5-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Two equivalents of trialkyl phosphite or dialkyl phosphorofluoridite such as the phospholanes shown upthread, are reacted with one equivalent of a
tri-a-substituted nitrosoalkane or nitroalkane in ether at a low temperature.
[Edited on 5-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
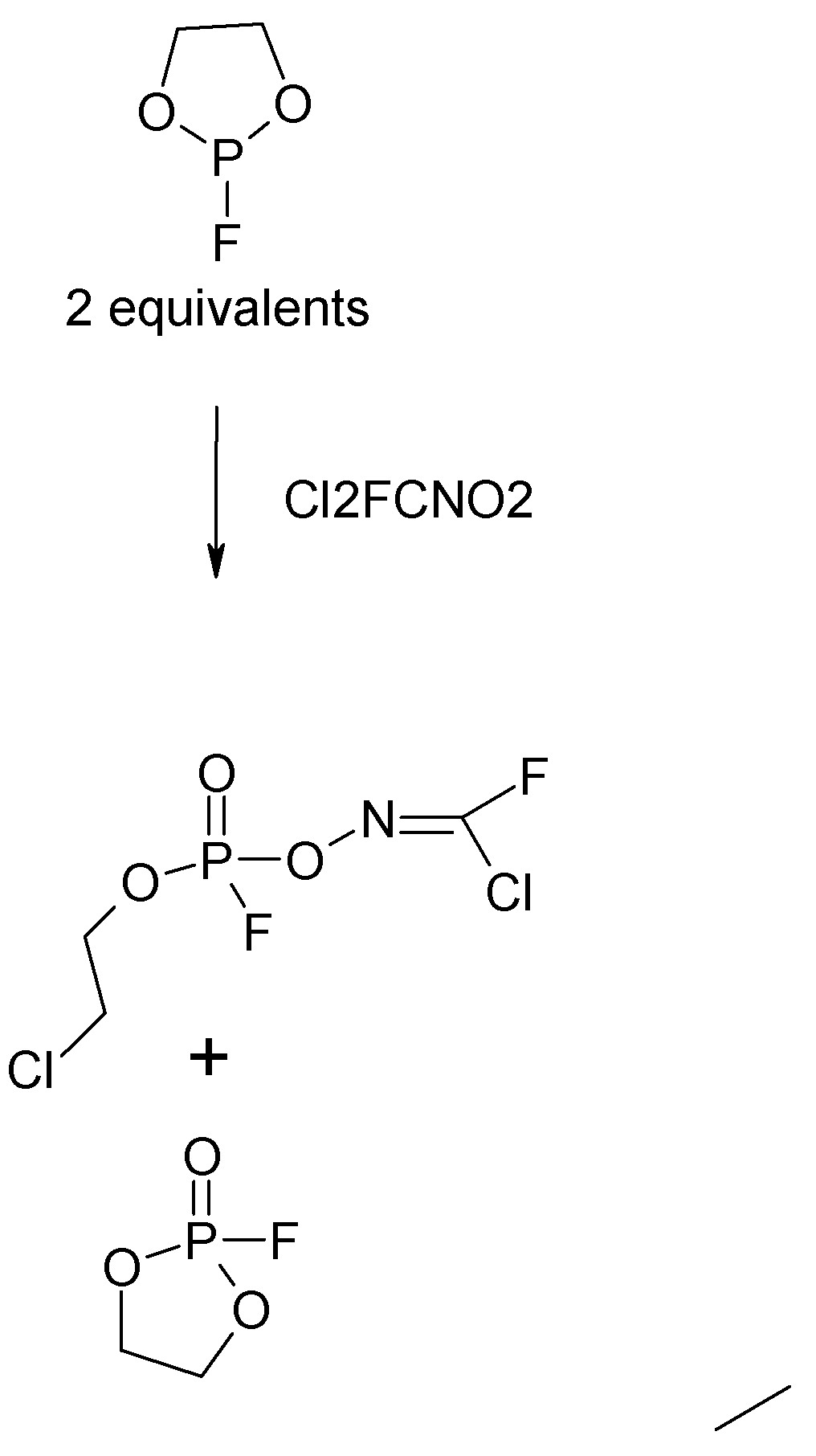
Now we are ready to fully understand the reaction of alpha-trihalonitroalkane or -nitrosoalkane with the fluorinated phospholane examples referred to
in the Samosa document and explained further in the paper posted upthread.
In the phospholane two of the P-O bonds are bridged by an ethylene to form the 5-membered heterocyclic ring.
Nucleophilic attack by the Cl from the nitro compound cleaves one R-O bond, opening the ring. The P-O bond become pi. The Cl rather than exiting as
an alkyl halide, remains on the beta position of the ethoxy chain. The second equivalent of fluorinated phospholane is likewise oxidized to
pentalaent P but without any bond cleavage.
So the reaction products are one equivalent of the oxime ester (novichok) and one equivalent of phosphonofluoridate.
Slightly simplified reaction graphic (intermediate omitted):
[Edited on 5-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
As the alpha-substituents on the nitro or nitrosoalkanes have to be Cl plus any combination of Cl, Br, F, and -CN or another psuedohalogen, there are
a limited number of possibilities.
Longer nitroalkanes than nitromethane are shown in the oxime ester literature but none in the five or six publicly disclosed novichok structures.
The other two substituents on P (besides the pi bonded O and the oxime ester) can be F, R = alkyl or OR = alkoxy. I would not rule out S containing
variations although none of the dissidents or emigres have mentioned any such.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
In regard to the oxidation step of the rxn scheme above for trichloronitrosomethane, the oxidation reagent choices are rather unsurprising.
Rathke (one of the pioneering investigators of CS2 chlorination) reported a very slow two phase oxidation of Cl3CSCl with nitric acid.
Later workers found that carrying out the reaction in glacial acetic acid as mutual solvent allowed efficient production of trichloromethanesulfonyl
chloride. JACS 63, 1764 (1941)
Oxidation with 30% H2O2 in GAA gave a much better yield (78%) of product of better quality.
Peracetic acid required forcing conditions which were IMO rather hazardous.
The urea-H2O2 adduct was also used succesfully.
Another reagent that has been applied is calcium hypochlorite.
The trichloromethanesulfonyl chloride is a solid. It has been found to be a useful and succesful chlorinating agent.
See attached paper.
If preparation of Cl3CSCl is deemed undesirable, Kolbe teaches that the sulfonyl chloride can be prepared directly from the same precursor (CS2) by
chlorination with wet, rather than dry, chlorine. Ann., 54, 145 (1845)
If CCl4 is available it is reported to react under mild conditions with sodium dithionite (Na2S2O4) to give trichloromethylsulfinyl chloride. The
preparation of the sodium salt from this ought to be straightforward.
Zhang, Kirchmeier, and Shreeve, Inorg.Chem. 31 492-494 (1992)
Huang, Huang, and Chen, Huaxue Xuebao (1982) 42 1114 (C.A. 102, 78313 (1985))
but since we can't buy CCl4 and prepare it from CS2, this is less helpful than it looks. For mixed halonitroalkanes however it is highly significant.
[Edited on 6-7-2008 by Sauron]
Attachment: jo01067a600.pdf (128kB)
This file has been downloaded 1102 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The Prandtl paper with preparation of sodium trichloromethanesulfinate is attached. Most of the paper deals with reactions of phosgene oxime and
derivatives, which can be ignored. The procedure for the salt is paragraph 1 of the experimental section. The method of O.W. Loew is employed.
(Z.Chem., 12, 82 (1869) Trimethylsulfonic chloride, recrystallized from ethanol, is taken up in methanol and reduced with H2S. The solution is
neutralized with anhydrius sodium carbonate using Congo Red paper to indicate end point.
The procedure immediately following for conversion of the salt to trichloronitrosomethane using sodium nitrite and potassium nitrate and hot 20% HNO3
is facile but the product is contaminated with water after distillation and some loss occurs due to thermal decomposition. Therefore the Suttcliffe
procedure referenced above is superior. The dry sodium salt of allowed to react sans solvent with condensed nitrosyl chloride under autogenous
pressure, reaction is complete when the vessel warms to O C or above. The trichloronitrosomethane is anhydrous and yield is improved.
[Edited on 6-7-2008 by Sauron]
Attachment: Ber1932.pdf (454kB)
This file has been downloaded 1657 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
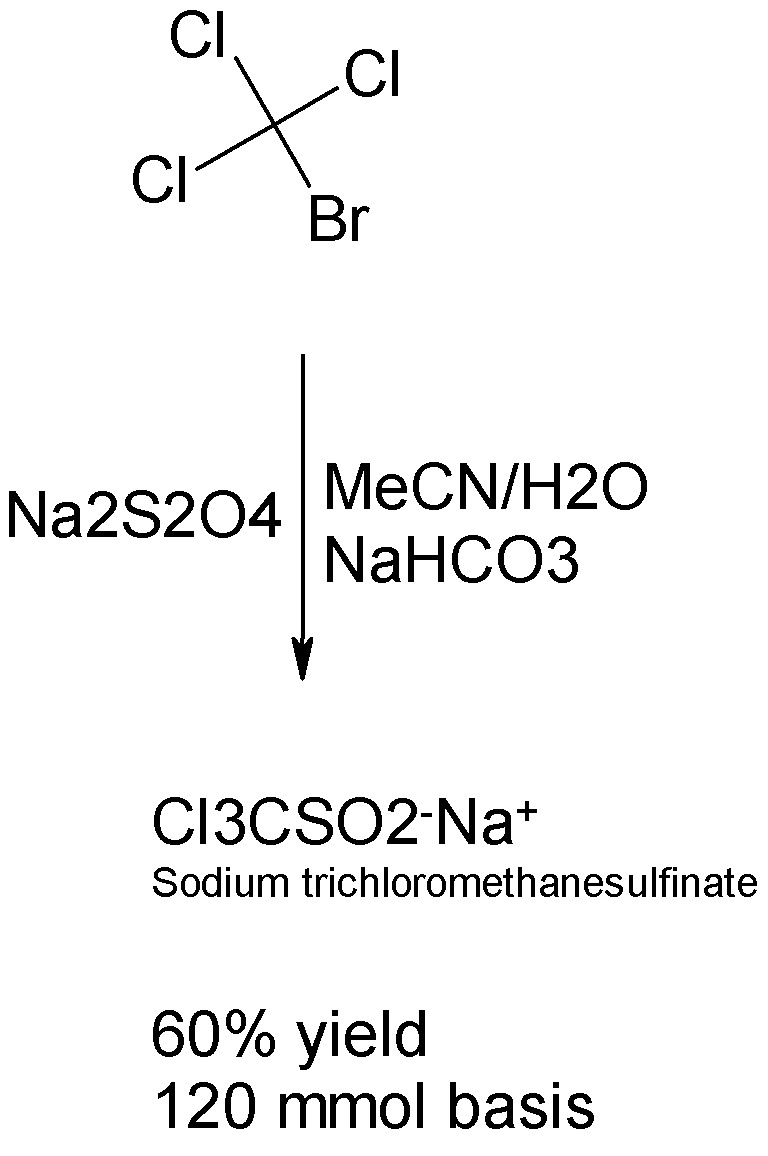
Hmmm. It appears that bromotrichloromethane (a heavy liquid) is commercially available and cheap, and not likely to be prohibited here (unlike CCl4.)
In this case a one step facile prep of sodium trichloromethanesulfinate is available.
The reaction between Cl3BrC and Na2S2O4 takes place in MeCN/H2O to form the sulfinate with sodium carbonate. See the Inorg.Chem article cited above
and attached below.
[Edited on 7-7-2008 by Sauron]
Attachment: ic00029a028.pdf (419kB)
This file has been downloaded 1228 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Note that the Na2S2O4 is sodium dithionite and NOT dithionate which is Na2S2O6. Sodium dithionate is also often called sodium hydrosulfite, which of
course - it isn't.
This certainly looks simpler and less costly than starting with CS2 and working with the nasty trichloromethyl sulfenyl chloride (perchloromethyl
mercaptan).
The observant reader will also take note that the same process can be applied to other perhalomethanes and so on, including Cl2FBr if you can get it
or make it. This opens up potential routes to the mixed halogen nitrosomethanes and homologs.
[Edited on 7-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
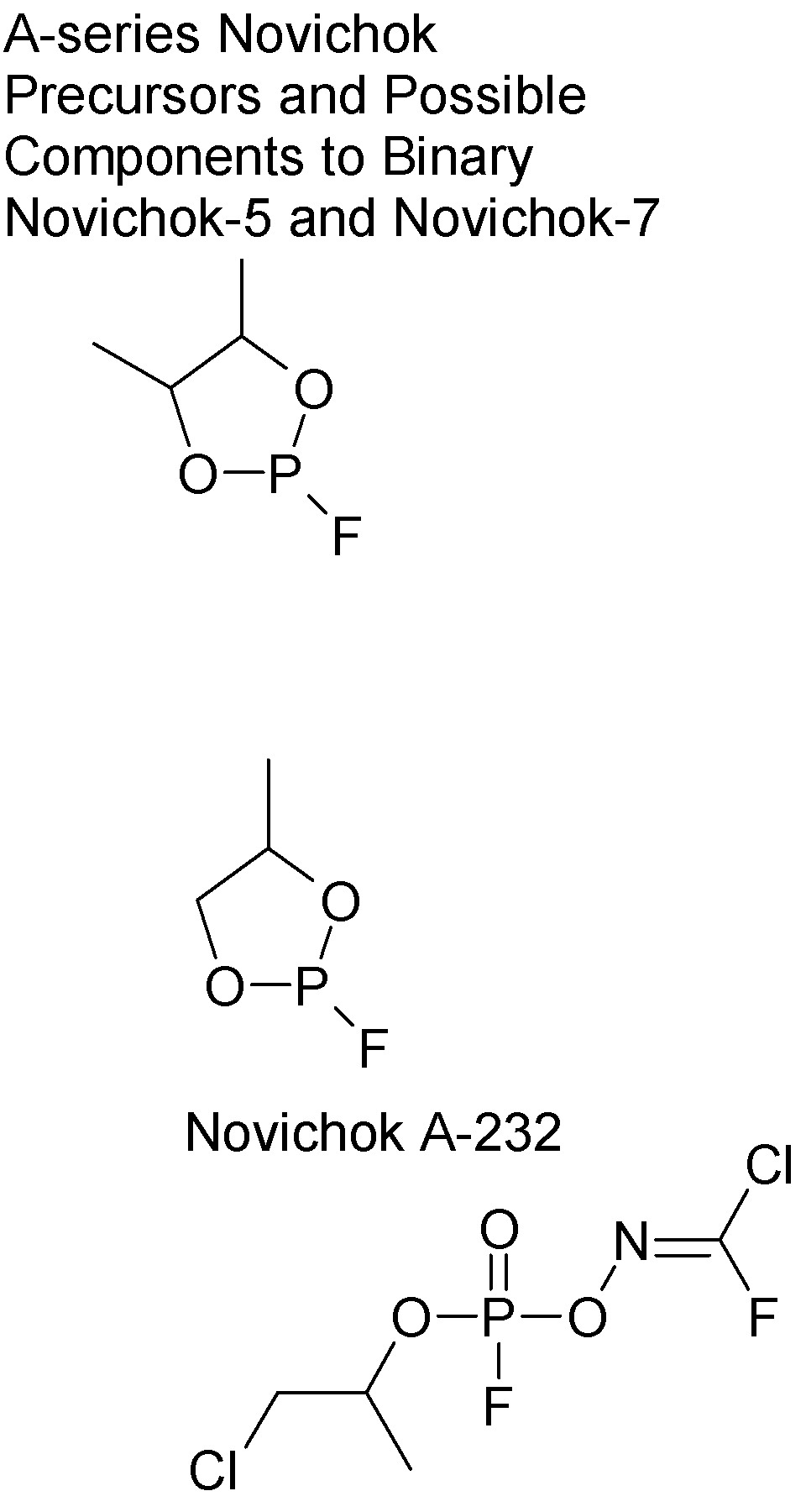
Errata and Corrections
A few errors to correct:
All references to A-240 and A-244 should read A230 and A-234.
There is also a reported A-232 Novichok intermediate in structure between those.
Some sources state that Novichok 5 and Novichok 7, said to be the most potent of the series and 5-8X more potent than VX, are the binary forms of two
of the structures shown. This has not been confirmed.
In the graphical reaction scheme from trichloromethanesulfenyl chloride to trichloronitrosomethane, the oxidative step to give
trichloromethanesulfonyl chloride can be performed by either nitric acid in glacial acetic acid, or, 30% H2O2 in GAA, the second method being
preferred.
The reduction of that product to trichloromethylsulfinic acid and conversion to the sodium salt is classically done with H2S followed by sodium
carbonate. The graphic shows, incorrectly, sodium in ethanol. NaOEt would react with alpha chlorines.
Graphic below shows two more fluorodioxaphospholanes along with A-232, one of the reported unitary Novichoks..
It is worth noting that there is no C-P bond in this molecule. This compound therefore is related to the fluorophosphates (e.g., Saunders' DFP) and is
NOT within the scope of the Chemical Weapons Convention.
The secondary alkoxy side chain, not being Me, Et, Pr, or 2-Pr, is also outside of the CWC "envelope" while still following the rules set down by
Saunders and Schrader for optimizing toxicity.
The P-O-N linkage is also outside of CWC's scope.
The requisite fluorodichloronitromethane or -nitrosomethane is also not CWC regulated (though chloropicrin itself is.)
The oxime ester does appear to be intended to frustrate NATO oxime-based OP therapies.
At first glance it does strain credulity to postulate that a DFP analog could match, exceed or even approach the toxicity of VX, as the two are
several orders of magnitude apart. However, this should not be a matter of mere surmise but of experimental determination and the synthesis is not a
difficult one.
This would need to be done in an appropriate facility and is therefore way outside of our status.
I would not at all be surprised if these compounds as shown could reach the toxicity of the G-series, as well as the versatility of engineered
persistence/nonpersistance, defeat of MOPP gear via cycloalkoxy moiety, etc. I do not know enough to intelligently comment about detection technology.
But if the oxime esters do in fact thwart known therapies then these could in fact be very dangerous military agents.
[Edited on 8-7-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The Russians call the reaction between phosphites and alpha-trihalonitro/nitrosoalkanes to form oxime esters of phospahtes the Allen Reaction,
although I have not seen any Western reference that indicates this to be an accepted name reaction. The JACS paper by J.Forrest Allen is posted
abovethread.
This reaction was an extension of reactions of phosphites with a-halocarbonyl compounds to give vinyl esters of phospahtes in a manner entirely
analogous to the prep of oxime esters.
Two earlier JACS articles by Allen and coworkers describing these reactions are attached, I combined them into a single pdf.
This allows a deeper understanding of the reaction central to novichok chemistry, to the extent that we know it.
See also that the employement of a dioxaphospholane as the phosphite was not lost on Allen. Ethyl ethylene phosphite is specifically mentioned.
[Edited on 8-7-2008 by Sauron]
Attachment: Allen.pdf (1.1MB)
This file has been downloaded 1542 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
It is apparent that the precursors for the required cyclic phosphite esters are simple common aliphatic glycols, namely
ethylene glycol
1,2-propanediol (propylene glycol)
meso-2,3-butanediol (2,3-BDO)
The latter two for the alleged components for Foliant 5 and Foliant 7 binary agent.
The preparation of these as the chloro compounds from the glycol and PCl3 equimolar in DCM is described in Lucas et al, JACS 72 5491-5497 (1950). I
will post this shortly.
Anyone skilled in the art will recognize numerous ways to convert the chloro compound to fluoro. Another option is to use PCl2F with the glycol in
first place. See Saunders.
The proper pronounciation of Novichok is "noweeshock" snd the term is not so universally recognized in defense circles. The code name Folient (or
Foliant) however gets an immediate heightening of attention. I have been assured, and reassured, by people who are in a position to know, that the
United States and NATO are equipped with Folient-impermeable protective gear, although I am told that this gear is incredibly costly and difficult to
manufacture. The transition to this apparel etc took place around the time of the first Golf War. I conclude therefore, that these newcomer agents
are very very real, and that their threat has been taken most sriously by the West for about the last 20 years.
That the former Soviet Union succesfully hoodwinked and neutered the Chemical Warfare Convention is now very clear. The protocols of CWC do not
encompass the precursors of the Folient agents nor the agents themselves in either unitary or binary form. The phosphorus containing precursors, and
the agents, contain no carbon-phosphorus bond. The dissident and defector accounts are IMO accurate although necessarily partial.
It has beena pleasure to tease out the chemistry of these newcomer agents.
The correct name for these compounds is 2-halo-1,3,2-dioxaphospholane (for the ethylene glycol case) while the others are 4-methyl and 4,5-dimethyl
substituted.
PF3 and PCl2F are pains to prepare and store, so so it is probably better to prepare the 2-chloro compounds then perform a halogen exchange. This
might be as simple as NaF in CHCl3 or CCl4. Again, see Saunders for analogous work on dialkyl fluorophospates.
[Edited on 28-9-2008 by Sauron]
Attachment: lucas.pdf (946kB)
This file has been downloaded 1390 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Backgrounder on the 2-chloro-1,3,2-dioxaphospholanes:
Here is the pertinent section from Volume 1 of the invaluable "Chemistry of Hetrocyclic Compounds" series (thanks, kmno4!)
Despite the jawbreaker name from Ring Index, these are just cyclic chlorophosphonite esters of simple 1,2-diols (glycols).
The trick therefore is to obtain PCl3.
[Edited on 28-9-2008 by Sauron]
Attachment: Pages from 1.pdf (711kB)
This file has been downloaded 1519 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The foregoing would be pretty useless without the references from its chapter, I have not bothered to cull this down to just the ones for that short
section.
Sorry for double posting but the two files were a bit large to be rolled into one so I am attaching them seperately.
[Edited on 28-9-2008 by Sauron]
Attachment: refs.pdf (1021kB)
This file has been downloaded 2155 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The trivial name for 2-chloro-1,3,2-dioxaphospholane is ethylene glycol phosphorochloridite. A googling of this name produced a Chinese paper on the
use of this reagent in phosphorylating sugars. The preparation from EG and PCl3 in 72% yield is described, CHCl3 or Et2O used as solvent. Reactants
were in equimolar proportions. The procedure appears to be a simplification of Lucas, q.v. Therefore I recommend employing Lucas precautions against
side reaction leading to linear esters, and also his admonition that the EG ester is very readily hydrolyzed and requires manipulation under anhydrous
conditions.
The 4-methyl and 4,5-dimethyl substituted glycol phosphorochloridites are reportedly much less readily hydrolyzed. These are from 1,2-propanediol and
meso-2,3-butanediol respectively.
[Edited on 29-9-2008 by Sauron]
Attachment: 150215-171-03-0100-p4.pdf (89kB)
This file has been downloaded 1579 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
2,3-butanediol is actually quite interesting. It can exist in three stereoisomers, 2R,3R, 2S,3S, and the meso form 2R 3S. The 2R,3R form exists in
nature and can be produced by fermentation.
Commercial 2,3-BDO is a mixture of the racemate and meso forms in approximately equal proportions (25:25:50 %). The racemic fraction can be isolated
in 97% purity by esterifying with Ac2O then holding at 4 C till the diacetate crystallizes out. The mother liquor is enriched in meso form. The
literature suggests that meso-enriched mixtures can be enriched in the racemate by distillation to about 70% racemate, this sounds like the meso form
can be removed relatively cleanly. The fractional crystallization can then be repeated.
The Lucas procedure calls for the meso form only, and while Aldrich does sell this it's only in 10 ml bottles and I shudder to contemplate the price.
Even the technical (racemate + meso) 2,3-BDO is expensive, like $300 a liter. Compare this with the dirt cheap propylene glycol (1,2-propanediol) and
I am forced to wonder why the Russians would give themselves a headache like this?
Well not such a headache as it turns out. Meso-2,3BDO can be prepared unequivocally from trans-2,3-butene oxide (epoxybutane) while the cis-isomer
gives only the dl form (racemate). Quite pure trans-2-butene is a commercially available gas and Org.Syn has the procedure for preparing the oxide,
while JACS has the procedure for converting tat to the meso-2,3-butylenediol.
I have now obtained the paper from Cytobiology on purification of dl-2,3-butanediol from mixtures with mes-2,3-butanediol. The former is a useful
cytoptotectant. However their procedure is not so useful in producing high purity meso-diol.
I also obtained another Lucas paper from JACS in vol 58 which details the preparation of pure meso-2,3-butanediol starting from mixtures of cis and
trans-2-butene, readily obtained by dehydration of n-butanol with H2SO4. The liquified 2-butenes are converted to their chlorohydrins, then to their
oxides, and fractionated. The epoxides are hydrolyzed with perchloric acid. trans-2-butene oxide gives exclusively meso-2,3-butanediol.
This procedure can be modified according to the method in Org.Syn. in which trans-2-butene oxide is prepared from trans-2-butene by means of 40%
peracetic acid. However, those authors used a spinning band column to purify their epoxide, and who has one sitting around?
Trans-2-butene is available commercially in cylinders. Having the pure (99%) alkene would obviate the fractionation of the opoxides.
In any case these papers amply demonstrate that practical meso-2,3-butanediol can be prepared with a reasonable amount of effort.
Here is the JACS paper, which is the best of the three IMO.
[Edited on 29-9-2008 by Sauron]
Attachment: lucas2.pdf (730kB)
This file has been downloaded 1296 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I see no particular need to post the Cytobiology paper unless requested.
As the only part of the Org.Syn. procedure that is pertinent is Note 2 I will simply quote it in entirety.
"2. trans-2-Butene oxide was prepared by appropriate modification of the procedure in Org. Synth., Coll. Vol. 4, 860 (1963). A 2-l., four-necked,
round-bottomed flask fitted with a mechanical stirrer, a 1-l. dropping funnel, an acetone–dry ice condenser, and a thermometer is charged with 1 l.
of 1,1,2,2-tetrachloroethane. The condenser is packed with dry ice and acetone, and the flask is cooled in a methanol-ice bath to −15°.
trans-2-Butene (153 g., 2.73 moles) (Phillips Petroleum Company, 99%) is distilled into the flask from a tared, chilled trap. Six hundred milliliters
of 40% peracetic acid (FMC Corporation), to which has been added 30 g. sodium acetate to neutralize the sulfuric acid present, is added to the stirred
solution from the dropping funnel over a period of 2 hours. The mixture is stirred at −15° for another hour, then allowed to warm to room
temperature. The mixture is poured into 1 l. of ice-cold water. The organic layer is separated, washed first with 10% sodium carbonate solution, then
with water, dried over magnesium sulfate, and filtered. Distillation of the filtrate through a 75-cm. spinning-band column gives 133 g. (68%) of
trans-2-butene oxide as a colorless oil, b.p. 52.5–55°."
It is worth noting that the same product from Lucas using a conventional fractionation and starting from a mixture of cis- and trans-2-butene oxides,
gave the same b.p. Therefore I suggest the spinning band distillation is a luxury and not a necessity.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Can i just say sauron i have thoroughly enjoyed following your investigation into these compounds and i appreciate you taking the time to compose and
post your musings, research, conclusions and the like.

|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks. I should qualify my remarks a little.
I am satisfied that Folient (or Foliant) is/was a real Russian/Soviet program and that is has been known to the West for several decades, and that
current MOPP gear was introduced to defeat it.
As to the structures, I cannot say with any certainty that the published structures, which are only three of what is supposedly a very large class of
compounds, are correct. The only way to find out would be to make them and see if you die! Unless you work at Edgewood or Porton Down etc. In which
case you probably know already one way or the other.
I have discussed the matter with a friend who formerly was one of the world's leading manufacturers of protective apparel includinf for CW and he
described to me the sea-change in MOPP (military organphosphorus protective) gear that took place around the time of the first Gulf War (Desert Storm)
and why.
The new clothing is complex, a total departure from previous technologies and very very expensive. He says it was developed specifically because of
the Novichoks.
Controversy is likely to continue over any possible connection between the so called Gulf War Syndrome and undisclosed Russian transfers of CW agents
to Saddam, specifically the vaguely defined Russian variant of VX. It is generally agreed that Saddam did NOT possess any Novichok agents.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
It's not illegal to discuss chemical weapons or share literature references, at least in the United States, where both I and the web host for this
site are located. I draw the line at discussing weaponization, but that is just part of the larger forum policy against discussing weapons
construction. Of course I also discourage people who are lazy or trolling and want to be spoon fed information, like the recently banned member
NovichokVX.
Edit: In case it is not already clear, I do not object to the chemical weapons discussions that have been conducted by Sauron, Ritter, Samosa, and
others over the years here. It remains an acceptable topic for discussion in the future too if it is reasonably academic.
[Edited on 12-3-2008 by Polverone]
PGP Key and corresponding e-mail address
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
I don't want any further off-topic discussion in this thread. Additional off-topic posts are subject to deletion.
Edit: it is not apparent now, but there was a long discussion in this thread about whether or not it's wrong to talk about chemical weapons in public.
The off topic material has been removed.
[Edited on 12-4-2008 by Polverone]
PGP Key and corresponding e-mail address
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
I've been avoiding these forums for a long time after some scares, but I'm really impressed with the level of discussion taking place--especially in
my particular area of interest ;-).
Let me digress a little bit: one thing that I have been looking into is not so much the chemistry of these compounds, but their actual
metabolism in the body. Questions such as: what is the mechanism of skin penetration? What is the mechanism and rate of binding to AChE and other
enzymes and neurotransmitters? Maybe a year ago, I wrote a report for my physical organic chemistry course on a Structure Activity Relationship for
the reaction between carbamates and acetylcholine. Unfortunately, that has been lost to a dead computer...
In any case, returning to "NTAs," as they are now called: at this point, it might be useful to compile a formal review of the public-domain literature
regarding these agents. Are there any forum dwellers who are fluent in Russian, perchance?
Speaking honestly: after completing my degree in Chemistry, I initially looked down on these forums as "not true science." However, with the
discussion taking place, it would be great if we could take the initiative to put more things into prepublication. I may be terribly mistaken, but I
don't know if such a comprehensive review of the Novichoks has been compiled yet--it might even be worthy of journal publication; or at least
something to add to your portfolio (let me tell you: the guys in Naval Research and Army Research are thirsty for people with any kind of background
or interest in this field--don't be deterred or afraid of big brother. He just might be on your side, so long as you're not aiding insidious types).
Sorry that I have nothing to add to this thread at the moment. I need to get back into the swing of things; I'll probably be spending more time using
the University databases to pull up some useful documents in the mean time.
Sauron--good job on the posts! Would it be possible for you to compile a list of your sources for others to glean over?
Cheers all.
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
simply RED
Hazard to Others
  
Posts: 209
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
According to docking and dynamic/kinetic analysis - this shoud be the most toxic of the series.
Extremely fast aging - "to perfection" - Enzyme-O-P(O)(OH)2.
The same toxicity should have the analogue where Sulphur-CH2- is substituted with -N=C(CH3)- .
Higher stability but less toxicity should have the sulphur substituted with oxygen and the fluorine substituted with -CH3.
[Edited on 9-3-2010 by simply RED]
[Edited on 9-3-2010 by simply RED]
[Edited on 9-3-2010 by simply RED]
When logic and proportion have fallen sloppy dead...
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I lost most of my interest in this series once I ascertained that the US and NATO has been well aware of them for c.25 years and tota;;y redesigned
MOPP gear and detection systems to deal with these by the time of Desert Shield, 20 years ago.
In short it is all old news, and can only be regarded as novel in comparison with preWWII G agents and early 1950s V agents'
[Edited on 9-3-2010 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
simply RED
Hazard to Others
  
Posts: 209
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
Of course they are old news!
They are just substituted VX.
The "drawback" of VX is that it contains P-O-R part, where R is Et or -CH2-CH(CH3)2 (Russian variety). This makes the enzyme inhibited by it
regeneratable with 2-PAM and such compounds, because it does not hydrolise (fast) further (aging) to P-OH. Aged enzyme (Enzyme-O-P(O)(OH)2 or
Enzyme-O-P(O)(OH)(CH3) is unregeneratable by any means.
If the -OR part in VX is : Pinacolyl alcohol, -O-CH=CCl2 (as in DDVP), -O-N=CCl2, etc - the enzyme ages much quickly and can not be regenerated. This
is the theory of the latest generation OPs like Novichoks. Also the H-O-N=CCl2 or H-O-N=CClF is very toxic and reactive on its own(a battlefield
toxin) and is released inside the synaptic cleft! This theory is published widely in the 1970s.
See attachment. Quantum/MM structural models may be wrong but most of the article is good.
Attachment: Aging.pdf (505kB)
This file has been downloaded 1106 times
Antidote or desactivator for these final generation OPs could be medium mass organic, polymer or biopolymer molecules designed to bind and desactivate
the OPs. Designed as key-keyhole to them - hydrolisisng the "tail" part, that is recognized by the acetylcholinesterase and the receptors. Such
antidotes could easily be enginnered with modern molecular modeling techniques (quantum calculations).
[Edited on 11-3-2010 by simply RED]
When logic and proportion have fallen sloppy dead...
|
|
|
| Pages:
1
2
3
4 |