| Pages:
1
2
3
4
5 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Such bicyclic systems where the ethylamine side chain is rotationally restricted by being incorporated in a cyclohexane ring are quite common among
the selective agonists at various monoamine receptors. Probably the most known is (R)-8-hydroxy-DPAT, due to its use as a selective 5HT1A agonist.
Note that the stereochemistry of pramipexole is R, thus the opposite of the most active amphetamine enantiomer indicating a completely unrelated
mechanism of action (D3 and other DA receptor agonism vs. DA&NE release).
Edit: -------------------------------------------------------------
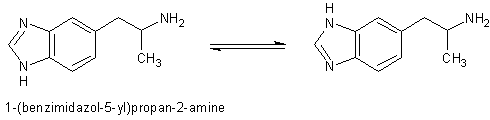
I mentioned earlier I would provide the references for 1-(benzimidazol-5-yl)isopropylamine. Here are the two references with abstracts that I could
find by searching for its structure and that of its tautomere. Anybody able to provide the full papers is welcome to do so.
Evaluation of the NE releasing ability of 1-(benzimidazol-5-yl)propan-2-amine ("decarboxylated I") in heart and brain:
| Quote: | Effects of DL-3-(5-benzimidazolyl)-2-methylalanine on brain and heart catechol amines. I. Depleting effects.
Zenker, N.; Morgenroth, V. H., III; Wright, J.; King, J. W.; Arnett, C. D.
Biochemical Pharmacology, 25 (1976) 585-589.
Abstract: DL-3-(5-benzimidazolyl)-2-methylalanine (I) [58894-60-3] (87.6 mg/kg, i.p.) decreased rat brain and heart catechol amines.
The releasing potency of decarboxylated I and the inability of I to lower heart norepinephrine [51-41-2] in the presence of a decarboxylase inhibitor
showed a decarboxylation product of the amino acid was responsible for the release of norepinephrine by I. When I and then a monoamine oxidase
inhibitor were administered to reserpinized rats, the level of brain amines remained const., indicating inhibition of norepinephrine synthesis in
vivo; the activity of tissue tyrosine hydroxylase [9036-22-0] in I-treated rats confirmed this inhibition. |
Synthesis starting from p-nitroamphetamine:
| Quote: | Medicinal agents in the series of beta-phenylisopropylamine derivatives. VI. Benzimidazole analogs of beta-phenylisopropylamine.
Piotrovskii, L. B.; Kudryashova, N. I.; Khromov-Borisov, N. V.
Khimiko-Farmatsevticheskii Zhurnal, 9 (1975) 3-5.
Abstract: Aminopropylbenzimidazole (I, R = H) was obtained in 50% yield in 6 steps from p-O2NC6H4CH2CHMeNH2 by redn., acetylation, nitration,
deacetylation, redn., and cyclization by HCO2H. Addnl. obtained was 60% I (R = Me). I have potential sedative activity (no data).
|
[Edited on 4-3-2007 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
Bravo, excellent material!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Well, here we go with another potential empathogen, 1-(indol-6-yl)isopropylamine:
Pharmacology related references with abstracts:
| Quote: | Synthetic indole compounds. V. Syntheses of indoles with (2-aminoethyl)-, (2-aminopropyl)-, or alkanolamine side chains on the six-membered
ring.
Troxler, Franz; Harnisch, A.; Bormann, G.; Seemann, F.; Szabo, L.
Helvetica Chimica Acta, 51 (1968) 1616-1628.
Abstract: 4-, 5-, 6-, and 7-Cyanoindoles are converted in excellent yields into the corresponding formylindoles by sodium
hypophosphite/nickel according to the method of Backeberg and Staskum (1961). Condensation of these formylindoles with nitromethane or nitroethane
affords the related nitrovinylindoles, which are reduced to the title aminoalkylindoles by LiAlH4. On the other hand, 5-chloroacetylindole is
aminated by reaction with various secondary amines, and the amino ketones formed are reduced to the corresponding 5-(2-amino-1-hydroxyalkyl)indoles.
Friedel-Crafts condensation of 1-acetyl-7-hydroxyindoline with ClCH2COCl yields 1-acetyl-4-chloroacetyl-7-hydroxyindoline (I), which is transformed
into indoline derivs. carrying a 2-amino-1-hydroxyethyl side-chain in position 4.
Effect of an indole derivative, 6-(2-aminopropylindole), on the general and coronary hemodynamics of the intact dog.
Maxwell, G. M.
Experientia, 20 (1964) 526-527.
Abstract: cf. CA 54, 4912d; 58, 8327a. Measurements were performed on dogs that were injected with the title compd. (total dose of
0.5 mg./kg., 3 injections at 5 min. intervals). The drug has little effect upon respiration. The increase in blood O and the maintenance of satn.
are due to the increase in hemoglobin, which is probably due to splenic contraction. The parallel trends in cardiac output and systemic pressure
increase both pressure and flow work, but maintain vascular resistance at control levels. The increase of coronary flow is modest and coronary
vascular resistance is unchanged. The increase in myocardial O extn. is a function of the increase in arterial O. |
Patents on 1-(indol-6-yl)isopropylamine by Hofmann and company:
| Quote: | Verfahren zur Herstellung von neuen Indolderivaten.
Hofmann, Albert; Troxler, Franz.
CH442304
Verfahren zur Herstellung von neuen Indol-Derivaten.
Hofmann, Albert; Troxler, Franz.
CH442303
Nouveaux dérivés de l'indole et leur préparation.
Hofmann, Albert; Troxler, Franz.
FR1344579 |
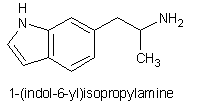
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
longimanus
Harmless

Posts: 17
Registered: 28-1-2007
Member Is Offline
Mood: No Mood
|
|
1-((S)-2-Aminopropyl)-1H-indazol-6-ol: A Potent Peripherally Acting 5-HT2 Receptor Agonist with Ocular Hypotensive Activity
Jesse A. May, Anura P. Dantanarayana, Paul W. Zinke, Marsha A. McLaughlin, and Najam A. Sharif
J. Med. Chem. 2006, 49, 318-328.
PDF
Abstract:
Serotonin 5-HT2 receptor agonists have been identified as a potential new class of agents for the treatment of ocular hypertension and glaucoma. The
initially reported tryptamine analogues displayed either poor solution stability, potent central nervous system activity, or both of these undesirable
characteristics and were unacceptable for clinical evaluation. A series of 1-(2-aminopropyl)-1H-indazole analogues was synthesized and evaluated for
their suitability for consideration as clinical candidates. 1-((S)-2-Aminopropyl)-1H-indazol-6-ol (9) was identified as a peripherally acting potent
5-HT2 receptor agonist (EC50 ) 42.7 nM, Emax ) 89%) with high selectivity for the 5-HT2 receptors relative to other serotonergic receptor subtypes and
other families of receptors and has significantly greater solution stability than R-methyl-5-hydroxytryptamine. Additionally, 9 potently lowers
intraocular pressure in conscious ocular hypertensive monkeys (-13 mmHg, 33%); this reduction appears to be through a local rather than a centrally
mediated effect. Compound 9 appears to be an excellent 5-HT2 receptor agonist for conducting further studies directed toward a clinical
proof-of-concept study for this class of ocular hypotensive agents.
Now what is important in the context of this thread are the results of the behavioural tests. | Quote: | Head-twitch induction in rodents, a stereotypical behavior induced by stimulation of central 5-HT2A receptors, has been used to assess the potential
side effects of serotonergic compounds. A good correlation has been observed between the rodent head-twitch response for a
compound and its ability to invoke a hallucinogenic response in man. However, this response has been shown to be modulated by agonist activity
at other receptors, such as 5-HT1A and 5-HT2C, so the results of this assay must be viewed with some caution if other functional data are not
available. Selected compounds from the present study along with the well-known centrally active standards 1(R-DOI)
and 5-methoxy-R-methyltryptamine (19) were evaluated for their ability to induce a head-twitch response in mice following subcutaneous administration
(Table 6). The effective dose required to produce a mean of five head twitches (ED5) during the 10-min scoring period was determined by interpolation
of the results from bracketing doses (see Supporting Information); this value was used to provide a relative ranking of tested compounds. The responses observed for 1 and 19 in this assay, ED5 ) 0.13 and 1.0 mg/kg, respectively, were consistent with the reported
human hallucinogenic dose for these two compounds, 2.3 and 2-4 mg, respectively. No perceivable response was noted in this assay for 2 (5-hydroxy-R-alpha-methyltryptamine) at doses ranging from 3 to 30 mg/kg, which is consistent
with the lack of hallucinogenic activity noted for this compound. As anticipated from the permeability data, 12 ((S)-2-(6-Methoxyindazol-1-yl)-1-methylethylamine fumarate) showed a
high level of headtwitch response, comparable to that of 1, with an ED5 of 0.16 mg/kg...
...The 7-methyl (14) and 7-chloro (16) compounds (i.e. 1-((S)-2-Aminopropyl)-7-methyl-1H-indazol-6-ol and
1-((S)-2-Aminopropyl)-7-chloro-1H-indazol-6-ol; yes, that's true - the phenolic compounds!!!) had ED5 values of 0.32 and 0.56 mg/kg,
respectively,... |
My comments
So, the indazole analogue of 5-MeO-AMT (alpha,O-DMS in TiHKAL) is about 6x more potent than the indole and equipotent to R-DOI. The synthesis
procedures suggested in the article are not the type of OTC-kitchen chemistry but still possible. 6-Methoxy-1H-indazole is commercially available and
that could make things a little easier (probably the authors started with 6-HO just because they wanted to end with a periferaly acting compounds).
For those interested in biochemistry it appears that "the tryptophan synthase alpha 2 beta 2 complex from Escherichia coli has been found to catalyze the beta-replacement reaction of L-serine with
indazole" to the indazole analogue of tryptophan (BIZA).
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Thanks for posting that paper, but at first glance it does not look particularly promising. Some things are however quite interesting in the view of
SAR theory. Yet these compounds were already in the patent literature by the same authors (WO0170702) as well as another group (US2004142998).
Isotryptamines (2-(indol-1-yl)ethylamines) and especialy their 2,3-dihydro derivatives are well known to have greater affinity for 5-HT2C over 5-HT2A
receptors. Following this SAR direction it is not strange that 1-((S)-2-aminopropyl)-1H-indazol-6-ol and its derivatives also have an affinity for
5-HT2C greater than 5-HT2A receptors. Compounds with this kind of unfavorable 5-HT2C/5-HT2A selectivity are not very promising as psychedelics
(quipazine is one such classical compound). Only the compound 14 appears to be a potential psychedelic (Ki = 1.8 nM for 5-HT2A vs.
3.6 nM for 5-HT2C; yet with such a logP it is probably too much peripherally active). I have no idea why this one deviates from the series. Moreover
due to the phenolic 6-hydroxy group the penetration of these compounds in the CNS is poorer as it would be in their 6-methoxy counterparts (here the
position 6 is equivalent to position 5 in tryptamines).
PS: It seems a pretty straightforward thought that the indazole is an effective bioisostere for indole in tryptamine derived serotonin receptors
agonists. Especially as an isostere in tryptamines, but I'm not aware of any 2-(indazol-3-yl)ethylamines having been tested for 5-HT2A receptor
affinity. I wish someone could dig out one such study. There are only some old references on the topic:
J. Am. Chem. Soc., 79 (1957) 5242-5245.
J. Am. Chem. Soc., 80 (1958) 965-967.
J. Am. Chem. Soc., 79 (1957); 5245-5247.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
longimanus
Harmless

Posts: 17
Registered: 28-1-2007
Member Is Offline
Mood: No Mood
|
|
I've worked long enough with animals to know that behavioural tests could not be trusted without serious analysis. However, if these these tests are
available then I'd prefer them in front of binding data. And here three compounds were active in the head-twitch.
Comment on receptor selectivity as predictor of hallucinogenic activity:
Yes truely, both DOI and LSD are a little more selective to 2A than 2C - respectively, 0.65:4.0 and 3.5:5.5 (in nM).
And, yes, quipazine is not selective agonist with pKi 2A:2C of 6.4:6.4 (or 6.9) (data from Organon).
However, the so famous S,S-dimethylazetidine analogue of LSD is more selective to 2C - Ki values of 8.3 and 6.5 nM for, respectively, 2A and 2C - and
still active at the discrimination study.
I've always had another hypothesis - partial agonism or at least specific modus of activation of the recetor (there is some literature data on the
asynchronous firing of serotoninergic neurons in presence of hallucinogenic drugs). Both DOI and LSD are ~ 3x less effective than 5-HT; still (data
from article above) the hallucinogenic 5-MeO-AMT is as effective as 5-HT.
I am a little puzzled.
[Edited on 22-8-2007 by longimanus]
|
|
|
dedalus
Hazard to Self
 
Posts: 60
Registered: 13-4-2007
Location: New York
Member Is Offline
Mood: No Mood
|
|
A fascinating discussion
...that I really lack the wit to add to, much, except to say:
The MPTP/induced Parkinsonism fiasco was a great boon to Parkinson's disease researchers, but the unwitting experimental subjects wound up royally
messed up.
I'd think about a hundred thousand times before ingesting anything on the basis of rat studies...I hope to contribute to Science, but not by being the
victim of the next diabolically selective neurotoxin.
|
|
|
longimanus
Harmless

Posts: 17
Registered: 28-1-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | | but I'm not aware of any 2-(indazol-3-yl)ethylamines having been tested for 5-HT2A receptor affinity. I wish someone could dig out one such study.
|
The compounds have been developed - or at least most of the 5-substituted and 1-Me derivatives of the AMT
analogue: for example 1-(5-methoxy-2H-indazol-3-yl)propan-2-amine
The fact that the depositors list pharmacologicaly active compounds in their database (not very large one - 24,000 or so) means that these indazoles
are evaluated, found active and data has been published. However, I couldn't find anything yet.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I'm not saying that compounds with 5-HT2A agonism weaker or similar to their 5-HT2C agonism would not be psychedelics. What I was trying to say is
that I think such compounds would be "qualitatively inferior". This could just as well be another prejudice since I never experienced quipazine and
can't really say what is it that I think would be qualitatively inferior. Also, we can not really say how selective these compounds are in activating
the 5-HT2A/2C receptors based only on their affinity since we don't know their efficiency in activating the second messenger production (they might
just as well be partial agonists). Furthermore, beyond the receptor selectivity and affinity, the other most important factor is the agonist directed
trafficking properties which can differ widely among different structures (not only we know these properties only for a limited number of compounds,
we don't even know for sure which one of the second messengers produced by the 5-HT2A receptor complex is actually involved in the psychedelic
phenomena).
I would not trust the head-twitch test or any other test based on reflex-like behavior as an indicator of psychedelic activity. That is barely any
more certain than the hyperthermic effect in rabbits or whatever other primitive test based on physiological response that was ever used. The only
data I would rely upon is a generalization study on rats trained to discriminate LSD, DOB or similar. This is the closer we can currently get in
asking a rat: "Are you tripping?" But in absence of such approach I find the affinity data the only truly useful information.
From what I gather in that paper I would still rather opt for the 2-(indazol-3-yl)ethylamines, especially the ones with a 5-methoxy and a small
hydrophobic group (Me, halogen, etc.) at position 7 (equivalent to the para position in the PEA psychedelics). The 2-(indazol-1-yl)ethylamines might
be an interesting curiosity to pursue, but first things first (just like tryptamines come before isotryptamines...). It is also worthy to consider the
possibilities that unsubstituted 1-(indazol-3-yl)isopropylamine and 1-(indazol-1-yl)isopropylamine might also have monoamine releasing abilities in
addition to 5-HT2 receptors agonism.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
longimanus
Harmless

Posts: 17
Registered: 28-1-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | | This is the closer we can currently get in asking a rat: "Are you tripping?" |
Now this is one of the
problems with pain also. We, people in pain physiology and pharmacology research, like to ask ourselves and each other a very difficult question -
"How are you sure that the animal experiences pain?". If pain includes emothional component and we cosider animals to be "devoid of emotions" what
dives us the right to determine their experience as pain? (Of course rats have emotions - one study showed the existance of empathic feelings, or at
least corresponding behaviour; that means one could test for MDMA analogues in rat  ) But how can you be sure that what the animal experiences on LSD is hallucinations (or at least sensory distortions) and not nausea or warmth (both
could be true)? After all psychedelics are ineffective in conditione-place preference. ) But how can you be sure that what the animal experiences on LSD is hallucinations (or at least sensory distortions) and not nausea or warmth (both
could be true)? After all psychedelics are ineffective in conditione-place preference.
| Quote: | | not only we know these properties only for a limited number of compounds, we don't even know for sure which one of the second messengers produced by
the 5-HT2A receptor complex is actually involved in the psychedelic phenomena |
Also do not forget the facts
that:
1. LSD effects are experienced hours after the plasma concentration has dropped below the active range.
2. Even after i.v. application about 1-2 h are needed for fully developed effects of this compound.
If you ask me - there's something with receptor desensitisation and/or protein synthesis.
| Quote: | | especially the ones with a 5-methoxy and a small hydrophobic group (Me, halogen, etc.) at position 7 |
I
thought that only 7-Me and 7-MeO and not Et or Br were behaviouraly active. Otherwise a very nice idea.
[Edited on 23-8-2007 by longimanus]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
For me pain includes more of a cognitive component than whatever emotional component. A burn would hurt much less if I would not be able to use
language and with it think so much about the pain. Since rats don't have their cognition enhanced by speech, one could argue their sense of pain is
different than ours. Kind of like children who only react to mosquito bites after they learn to speak and realize the annoying aspect of the itching.
But in drug discrimination tests you don't directly ask the rat to tell you how that drug feels like with the presumption it can experience the drug
similarly as humans would. Since rat's cognition is not based on language we can only guess how it feels to them. I would imagine it's something
completely different anyway. In reality with these tests it is completely irrelevant how a drug feels like to the animal, because what the rat tells
us is how similar a certain test drug is in comparison to the training drug. This therefore does not depend on the actual interspecies communalities
of the effects. That's why I think the generalization studies are the most exact animal test possible for testing potential psychedelic drugs. Just
think about it. How could a human know a drug has any psychedelic effects unless he experienced the psychedelic phenomena previously? We know what
that is only after we have been trained to discriminate it from other types of experiences. Just like those rats are.
Of course, in practice it is much simpler to just try the new compound by yourself, but when one does academic type of research this is not an option.
| Quote: | | I thought that only 7-Me and 7-MeO and not Et or Br were behaviouraly active. Otherwise a very nice idea. |
From the well known pharmacophore of PEA&tryptamines as 5-HT2A agonists it is clear that the equivalence between the two structural classes are:
PEA ortho-methoxy = tryptamine 5-methoxy
PEA meta-methoxy = tryptamine pyrrole ring
PEA para-substituent = tryptamine 7-substituent
For effects of tryptamine substitution at position 7 see:
Studies on Several 7-Substituted N,N-Dimethyltryptamines.
R. A. Glennon, E. Schubert, J. M. Jacyno.
J. Med. Chem. 23 (1980) 1222-1226.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
longimanus
Harmless

Posts: 17
Registered: 28-1-2007
Member Is Offline
Mood: No Mood
|
|
One dosn't need to understand any language to comprehend pain - there are cases of anencephals who, although without language centers (and almost all
other parts missing) are able to feel the pain and express aversive behaviour; this may not br convincing but there's another case - the case of Terri
Schiavo - brain damage; "When she emerged from the coma, Schiavo regained a sleep-wake cycle, but did not exhibit repeatable and consistent awareness
of herself or environment."; still, "The legal record, however, shows Michael's (husband's) claim that Terri felt
pain.". Language is only a tool for the expression of pain, just like vocalisation in rats. And for the understending of pain a developed brain is
needed, therefore the learning ofspeaking is accompanied by better comprehension of pain - after all if one tries to feed one's 1-month old baby with
hot milk it will deffinately vocalise for the next hours; however this is a stronger noxious stimulus than insect bites.
I agree that discriminative studies have shown good results. But still I'm not sure that what they feel isn't nausea, for example. Laboratory animals
have never been exposed to emetics how could they discriminate between regular emetics and hallucinogens with emetic action - training with, for
example, emetin or apomorphine are needed. Of course I understand that this is almost cerainly not true, that discrimination studies, especially if
combined with evaluation of the behaviour of the animals, is a good test for hallucinogens (whateverthey feel) . Still, there is place for doubts.
| Quote: | Studies on Several 7-Substituted N,N-Dimethyltryptamines.
R. A. Glennon, E. Schubert, J. M. Jacyno.
J. Med. Chem. 23 (1980) 1222-1226. |
Yes, 7-Br and 7-ethyl substituted 5-MeO-DMT only partially generalised
to te parent compound and disrupted behaviour at higher doses.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Here is a literature search for compounds of the 2-(indazol-3-yl)ethylamine type, its alpha-methyl derivatives, ring substituted and similar:
The relative potency of 5-hydroxytryptamine-like substances.
Bertaccini, G.; Zamboni, P.
Archives Internationales de Pharmacodynamie et de Therapie, 133 (1961) 138-156.
Abstract: The relative potencies of 78 analogs of tryptamine and 5-hydroxytryptamine (I) were detd. on the smooth muscle of estrus
rat uterus, guinea pig ileum, rabbit ear, and the heart of Helix luconum. The most active compds. were I and 5-hydroxy-N-methyltryptamine. The chem.
structure changes accompanying decreased pharmacol. activity in rat uterus included: (a) shifting of the phenolic OH group from position 5, (b)
substitution on the indole nucleus of groups other than OH, (c) etherification of the phenolic OH with glucuronic or sulfuric acids, (d) lack of any
phenolic OH group, (e) shifting of the side chain from the 3 to 2 position. The effect of alkyl substitution on the amino N was variable but usually
decreased with increase in size and no. alkyl groups. Substitution of 1 Me group on the alpha C had a variable effect but with 2 Me or 1 Et group
substitution there was a decrease of activity. Introduction of an OH group on the beta C of the side chain gave almost total loss of activity.
Indolealkylamines with a single C atom lateral side chain had little activity. Indazolealkylamines showed a I-like activity. Results with guinea pig
ileum generally paralleled these, while results on Helix heart were less consistent. None of the examd. compds. had marked antagonistic activity.
The most active antagonist for rat uterus was N,N'-dipropyltryptamine.
Antidepressant agents. III. Aza analogs of etryptamine.
Carnmalm, Bernt; Misiorny, Alfons; Ross, Svante B.; Stjernstrom, Nils E.
Acta Pharmaceutica Suecica, 11 (1974) 196-200.
Abstract: Etryptamine analogs RCH2CHEtNH2 [R = imidazo[1,2-a]-pyrid-3-yl (I), 1H-indazol-3-yl (II), 1H-pyrrolo[2,3-b] pyrid-3-yl
(III)] were prepd. by treating RCHO with PrNO2 and reducing the RCH:CEtNO2. II and III were less active monoamine oxidase inhibitors than etryptamine
and I was inactive.
Synthesis and radioprotective activity of -(3-indazolyl)ethylamine derivatives.
Rao, Erchang; Li, Meijia; Wang, Rongxian; Zhuang, Xianglian.
Yaoxue Xuebao, 22 (1987) 426-432.
(synthesis of 2-(5-MeO-indazol-3-yl)-1-dimethylaminoethane and similar)
US5276051 : Arylethylamine compounds.
US2003171418 : Preparation of pyranoindazoles and their use for the treatment of glaucoma.
WO2002098860 : Novel fused indazoles and indoles with 5-HT2 receptor activity, and their use for lowering of intraocular pressure in the
treatment of glaucoma.
WO2001070701 : Preparation of substituted 3-(2-aminopropyl)-5-hydroxyindazoles and related compounds for treating glaucoma.
WO2002098350 : Pyranoindazoles with 5-HT2 receptor activity, and their use for lowering intraocular pressure in the treatment of
glaucoma.
WO2000012482 : Preparation of (indazol-3-yl)alkaneamines as 5-HT2 receptor ligands.
(contains affinity and efficiency data for 5-HT2A and 2C receptors for two compounds: 5-chloro- and 5-bromo-indazol-3-ylisopropylamine)
US5385928 : Tetrahydrobenz[c,d]indazoles as serotonin agonists, compositions and use.
[Edited on 25/8/2007 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Filemon
Hazard to Others
  
Posts: 126
Registered: 26-4-2006
Member Is Offline
Mood: No Mood
|
|
Do you know 1,2,3,4-tetrahydroisoquinoline and derived as 3-methyl-1,2,3,4-tetrahydroisoquinoline? I know little, I only know that they are
investigated to treat the narcolepsic (http://www.patentstorm.us/patents/6703392.html). it should be psicoactive because it is a phenylethylamine but anything interesting (I suppose).
[Edited on 2-9-2007 by Filemon]
[Edited on 3-9-2007 by Filemon]
[Edited on 3-9-2007 by Filemon]
|
|
|
longimanus
Harmless

Posts: 17
Registered: 28-1-2007
Member Is Offline
Mood: No Mood
|
|
Probably half of the natural alkaloids contain the phenethylamine and tryptamine skeletonin their molecules but most of them are not psychoactive. The
abovementioned patent desribes derivatives of 1-benzyl-1,2,3,4-tetrahydroisoquinoline (the skeleton of compounds such as papaverine, narceine,
laudanine, etc). The dervatives from that patent are suggested to be orexin antagonists and to be useful in the treatment of obesity (through
suppression of apetite) and narcolepsy. However, I am not aware of any of the psychoactive properties Filemon is refering to. Evemn more - orexin
antagonists appear to decrease spontaneous physical activity:
Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats
Kohji Kiwaki, Catherine M. Kotz, Chuanfeng Wang, Lorraine Lanningham-Foster, and James A. Levine
Am J Physiol Endocrinol Metab 286: E551-E559, 2004
Abstract:
In humans, nonexercise activity thermogenesis (NEAT) increases with positive energy balance. The mediator of the interaction between positive energy
balance and physical activity is unknown. In this study, we address the hypothesis that orexin A acts in the hypothalamic paraventricular nucleus
(PVN) to increase nonfeeding-associated physical activity. PVN-cannulated rats were injected with either orexin A or vehicle during the light and dark
cycle. Spontaneous physical activity (SPA) was measured using arrays of infrared activity sensors and night vision videotaped recording (VTR). O2
consumption and CO2 production were measured by indirect calorimetry. Feeding behavior was assessed by VTR. Regardless of the time point of injection,
orexin A (1 nmol) was associated with dramatic increases in SPA for 2 h after injection (orexin A: 6.27 ± 1.95 x 103 beam break count, n = 24;
vehicle: 1.85 ± 1.13 x 103, n = 38). This increase in SPA was accompanied by compatible increase in O2 consumption. Duration of feeding was increased
only when orexin A was injected in the early light phase and accounted for only 3.5 ± 2.5% of the increased physical activity. In a dose-response
experiment, increases in SPA were correlated with dose of orexin A linearly up to 2 nmol. PVN injections of orexin receptor antagonist SB-334867 were
associated with decreases in SPA and attenuated the effects of PVN-injected orexin A. Thus orexin A can act in PVN to increase nonfeeding-associated
physical activity, suggesting that this neuropeptide might be a mediator of NEAT.
Full Text (PDF)
[Edited on 3-9-2007 by longimanus]
[Edited on 3-9-2007 by longimanus]
|
|
|
Filemon
Hazard to Others
  
Posts: 126
Registered: 26-4-2006
Member Is Offline
Mood: No Mood
|
|
I have not affirmed that it is stimulating. I have not found information on 3-methyl-1,2,3,4-tetrahydroisoquinoline. But I have found
1,3-Dimethyl-1,2,3,4-tetrahydroisoquinoline it is neurotoxic (it causes parkinson).
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Filemon
it should be psicoactive because it is a phenylethylamine but anything interesting (I suppose). |
That is a very stupid and thoughtless claim to say the least!
I have already posted a thorough literature review with references on the psychoactive 1,2,3,4-tetrahydroisoquinolines elsewhere. I'm not sure but you
might find the rescued thread from the otherwise dead forum somewhere on the net if you UTFSE. In any case these compounds are completely off topic
here in this thread. I think it was already established this thread is about 2-(heteroaryl)ethylamines only.
|
|
|
-TheMadMen-
Harmless

Posts: 29
Registered: 29-5-2009
Member Is Offline
Mood: No Mood
|
|
I Am amazed sometimes at the threads and people in this forum. Even the topic of this post "theoretical amphetamine variants" how many of you people
are actually professional chemists? Or are you all just amateurs like myself? With an intense passion for chemistry?
As far as amphetamine chemistry goes, isn't it extremely well covered by now? I mean, alexander shulgin for instance, pioneered his findings of
phenethylamine chemistry in pihkal, as im sure your all aware. But what's with this bullshit theoretical talk of amphetamine analogs when everybody
knows that the two most mind-altering chemical backbones of all in regards to mammals and humans included, are either phenethylamines or tryptamines??
Infact, the most potent, mind-altering chemical of all, if you look at the phenethylamines, is amphetamine itself, the most simple, basic, non-clogged
phenethylamine there is, anymore side-chain editions and what not, just dulls the potency, but not necessarily the psychopharmalogical profile.
I Saw something interesting the other day, in some old patent, if you boil dexamphetamine freebase oil in the presence of chloral hydrate, you end up
with a molecular change on the amphetamine molecule, which makes it less potent, at least in the mental sense, but still provides superior appetite
suppressant abilities. A Good example of how pharmaceutical companies use to, or still do, try and modify basic things, to change there
pharmacological profile.
[Edited on 29-5-2009 by -TheMadMen-]
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
the pharmacuetical companies are always on the hunt for ways to push amphetamine under new patents by making salts of amphetamine that have'nt been
marketed before which at the end of the day are simply racemic or enatiomerically pure amphetamine salts.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
| Quote: | | As far as amphetamine chemistry goes, isn't it extremely well covered by now |
Not even a little bit. Even as of 1998 substances such as bromo dragon fly made there appearence with a dosage and strength comparable to that of LSD
and since then much stronger analogs that target the 5HTP-2a receptors have been synthesized .
| Quote: | | As far as amphetamine chemistry goes, isn't it extremely well covered by now? I mean, alexander shulgin for instance, pioneered his findings of
phenethylamine chemistry in pihkal, as im sure your all aware. But what's with this bullshit theoretical talk of amphetamine analogs when everybody
knows that the two most mind-altering chemical backbones of all in regards to mammals and humans included, are either phenethylamines or tryptamines??
Infact, the most potent, mind-altering chemical of all, if you look at the phenethylamines, is amphetamine itself, the most simple, basic, non-clogged
phenethylamine there is, anymore side-chain editions and what not, just dulls the potency, but not necessarily the psychopharmalogical profile.
|
This statement is highly misguided and seems to stem from your narrow areas of research. You seem to forget substances such as PCP and Ketamine,
Opiods, Tropine based substances such as Cocaine just to name a small few. Point being where as Phenethylamines and typtamines receptors have the
ability to basicly be receptor sluts and accept a whole range of variations this does not conclude that all drugs are based on these skeletons.
@Nicodem
Have you seen any instance in your research that would suggest the use of the structure present in 1-(indol-6-yl)isopropylamine to replace the
benzylfuran rings of the Bromo dragonfly variant? Ill generate a structure to give you an idea of what im proposing if needed but this text,
http://www.thevespiary.org/talk/index.php?action=dlattach;to...
States that the singlet hydrogen that is attached to the benzylfuran is what aids in the strong binding to the receptor and the indole variant should
at first glance form a simular potancy I would assume.
[Edited on 29-5-2009 by Sedit]
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
-TheMadMen-
Harmless

Posts: 29
Registered: 29-5-2009
Member Is Offline
Mood: No Mood
|
|
| Quote: | | This statement is highly misguided and seems to stem from your narrow areas of research. You seem to forget substances such as PCP and Ketamine,
Opiods, Tropine based substances such as Cocaine just to name a small few. Point being where as Phenethylamines and typtamines receptors have the
ability to basicly be receptor sluts and accept a whole range of variations this does not conclude that all drugs are based on these skeletons.
|
I'm talking more about "mind-altering" as such, while i agree, ketamine in general, and pcp, but more so ketamine, as i find it more fascinating,
seems to act more unique than any kind of tryptamine or phenethylamine or even tropane alkaloid, does it not? I Mean, the sensory-isolation that
ketamine provides, is well-documented, but what about it's effects on perception? Amphetamine in general, and the slightly less potent
methamphetamine, alter perception immensely, but not does as say, lsd, in which your perception of reality is altered, rather, the perception of
reality is heightened to such an extent by drugs like amphetamine, that you seem to gain a more rational, wide-scoped, non-forced view of the world,
you become like a tiger, or even a tick, a parasite, whereby the only thing on your mind as such, is to use whatever force nessecery to get what you
want. Even if it defies "normality", and so i'd consider that to be warping or changing your sense of perception to a certain extent, as by virtue of
who we are, humans, have an instinctive mindframe which is to basicallly agree with the "leader of the pack", and in this case, it's society and the
law and media, amphetamine reverses that instinct and makes you think for yourself, and possibly those around you with which you love. Like an animal.
Which is what makes it so addictive and appealing, or not to mention just the euphoria that it induces, that's what i love about it anyway, heh.
And let's not forget fentanyl, speaking of mind-altering things, and opioids/opiates in general, and as a matter of fact, even imidazopyridine's seem
to have some interesting psychopharmacological activity.
And then also ethanol, which is a poison, and so poisons not only your brain, but body too, as do other things, like toxic metals, some mentally
insane people on forums like psychonaut seem to think that poisoning yourself with such toxic elements is worthy of a "good trip", i think that they
are nutcases, i'm more interested in things that do the same, similiar, or if not better, by emulating the natural neurotransmitters/neurochemicals
that our brains handle on a normal basis, so the non-toxic way, as i'm sure you all are too, hehe.
|
|
|
-TheMadMen-
Harmless

Posts: 29
Registered: 29-5-2009
Member Is Offline
Mood: No Mood
|
|
It's all very interesting, however, and, baroque, as goes for all psychopharmacology it seems, cocaine for instance, so utterly different to
amphetamine in molecular structure, is indifferent in effect profile, while nicotine, with no seemingly profound psychopharmacological effects, seems
to incite itself on the conscious mind in it's addictive demands for more of it.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by -TheMadMen-  | ...
As far as amphetamine chemistry goes, isn't it extremely well covered by now? I mean, alexander shulgin for instance... |
Sedit already addressed this, I will add that Shulgin himself, in a talk he gave at Johns Hopkins a couple of years ago, seemed to feel that there
was plenty more to mined from the phenethylamines. Other structure systems are just getting to be rationally understood in their effects, there's
much left to be learned I suspect.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
| Quote: | | Amphetamine in general, and the slightly less potent methamphetamine, |
Not to knock your post Madmen because you appear to put some thought into them atlest but where do you get your information from? AMPH is somewhere on
the order of 1/10 the potancy of Methyl Amphetamine so once again I am slightly confused by this response.
| Quote: | | I will add that Shulgin himself, in a talk he gave at Johns Hopkins a couple of years ago, seemed to feel that there was plenty more to mined from the
phenethylamines. |
N_I speaks the truth and Shulgins is still to this day tinkering with his favorite substrates. One variation I am highly intrigued by is an image that
was leaked of Shulgins himselfs lab that showed a blackbord with an image on it that said "make me" under it. It appeared to denote IIRC a substituted
carboxylic acid moriaty attached to the alpha carbon and a methylene dioxy one on the benzene ring. This defies most means of normal binding that I
know of for other phenylethylamine structures and may point to other receptors of binding hence completly different effects.
| Quote: | And then also ethanol, which is a poison, and so poisons not only your brain, but body too, as do other things, like toxic metals, some mentally
insane people on forums like psychonaut seem to think that poisoning yourself with such toxic elements is worthy of a "good trip", i think that they
are nutcases, i'm more interested in things that do the same, similiar, or if not better, by emulating the natural neurotransmitters/neurochemicals
that our brains handle on a normal basis, so the non-toxic way, as i'm sure you all are too, hehe.
|
This is pretty much what they all do in one way or another including ethanol and PCP. Phencyclidine has its own receptors based all thoughout the body
for use in situations of cronic pain and death. This is one of the reasons that some people can be shot or have a limb severed in freak accidents and
not feel the pain of it for sometime after.
There are some very good pharmacology websites out there I suggest you look into them you will more then likely find there content truely fasinating.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
@Sedit perhaps he refers the case of substituted phenylisopropylamies where the N-methylated variants are noted as being somewhat less potent
as a hallucinogen this of course ignores the very significant qualitative change in the experience caused by N substitution of MDA.
@TMM Its nice to have a new member who has done some reading and bothers to spell properly and I don't want to discourage- but it is difficult to take
you seriously on an in depth SAR discussion when you have misunderstood something as basic as this.
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
| Pages:
1
2
3
4
5 |
|