| Pages:
1
2
3 |
Sulaiman
International Hazard
    
Posts: 3766
Registered: 8-2-2015
Member Is Offline
|
|
confused (Mg:Cu sulphate separation/re-crystalization)
The resurrection of this thread made me consider, just as an exercise,
re-crystalizing some solutions that I used for electrochemical cell (primary batteries) experiments,
both are presently saturated at c15 oC
. copper sulphate solution that is contaminated with an unknown ammount of magnesium sulphate
. magnesium sulphate solution that is contaminated with an unknown ammount of copper sulphate.
Although I have done re-crystalizations before I did not think about it much, I just followed a procedure.
Why would only the desired compound crystalize out ?
............................................................................
Solubility curves for MgSO4.7H2O and CuSO4.5H2O for reference, if needed.
Notice the curve for magnesium sulphate ... I'll think about that later 
Attachment: Cu_Mg_SO4.ods (19kB)
This file has been downloaded 401 times
Attachment: Cu_MgSO4.xls (10kB)
This file has been downloaded 402 times
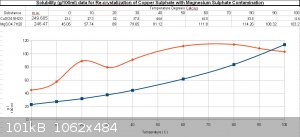
EDIT:
MgSO4.5H2O is a known substance, so to my simple mind, Mg and Cu could even form a double-salt / co-crystal / quasicrystal, why
not ?
[Edited on 16-7-2017 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
experimental
Harmless

Posts: 21
Registered: 9-7-2017
Member Is Offline
Mood: curious
|
|
Ok, I found a solution to the mixed copper sulfate and copper chloride crystals. Just added some distilled water and a small amount of sulphuric acid.
Then boiled the water like in an ordinary recrystallization, and the more volatile hydrochloric acid evaporated.
The recrystallized crystals were uniform in color, thus only copper sulfate remained, but probably with some H2SO4 contamination despite washing them
with cold water.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
If you want pure Copper sulfate for a test make it!
H2SO4 + 35% H2O2 + Copper
use a container that is much larger then the acid peroxide charge as this will foam and spew out Ozone, so do out side or in a good fume extraction
system, it will warm up plenty on its own as well.
You do not need a whole lot of peroxide either as it seems to behave like a catalyst in this reaction.
I used 50ml concentrated H2SO4 to 25ml of H2O2 then just dumped copper in till it stopped reacting, then I added 10ml more H2O2 and heated the
solution till no more copper would react (I used excess copper, so the H2SO4 was my limiting reagent)
let cool to room temp then placed in the freezer, filtered the solution in a paper filter then air dried then warmed to semi dehydrate them to ensure
any trace of the peroxide was decomposed. got a nice bottle of pure copper sulfate for testing my hydrazine sulfate with.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Per Wikipedia (https://en.m.wikipedia.org/wiki/Copper(II)_sulfate ), the copper(ll) sulfate pentahydrate is, to quote:
"soluble in methanol[1]
10.4 g/L (18 °C)
insoluble in ethanol, insoluble in acetone"
So dissolve the copper(ll) sulfate pentahydrate in a sufficiently large volume of CH3OH, and most other possibe impurities (MgSO4, FeSO4,...) should
precipitate out.
Decant and just let evaporate or apply heat.
This simply method does show, per precipitates, a rough idea of the level of impurities, which can be further measured. Unfortunately, it requires a
100 ml of methanol for each gram of the pentahydrate. One could do a few grams at a time only, and reuse the CH3OH, which is recaptured by
distillation.
[Edit] Per another source (see https://pubchem.ncbi.nlm.nih.gov/compound/Copper_II__sulfate... ):
from HSDB
1 g in 3 ml glycerine
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 748
from HSDB
15.6 g/100 cc methanol @ 18 deg C
Weast, R.C. (ed.) Handbook of Chemistry and Physics, 68th ed. Boca Raton, Florida: CRC Press Inc., 1987-1988., p. B-90
[Edited on 10-8-2017 by AJKOER]
|
|
|
18thTimeLucky
Hazard to Self
 
Posts: 51
Registered: 19-8-2017
Location: The one-and-only tea and crumpet land (UK)
Member Is Offline
Mood: 0 Kelvin and still won't crystallise from solution
|
|
I guess I am late to the crystal growing competition from last year?
I just started using ScienceMadness yesterday and decided to make an account today and I couldn't resist to post some pictures of a copper sulfate
crystal I have been growing since January 2017 as my first post.
This thread seems to have been semi-resurrected so I hope I can please atleast one person with some blue beauty! (its mass is 46g)
It has some smaller crystals growing out of it because I am an idiot and recently replaced the copper sulfate solution but I was not thinking and used
hot water so when it cooled down I got crystals everywhere as you can imagine, maybe I thought I was doing a recrystallisation or something...
If anybody is interested in my process, just ask! I have plenty of pictures from almost every month since I have started growing it.
I hope this isn't too different from the thread which is more aimed at purifying copper sulfate by recrystallisation.
(and yes that is an upside down curry paste jar, no expense is spared for you guys)
![IMG_7162[1].JPG - 1.5MB](https://www.sciencemadness.org/whisper/files.php?pid=490654&aid=60907) ![IMG_7164[1].JPG - 1.4MB](https://www.sciencemadness.org/whisper/files.php?pid=490654&aid=60909)
[Edited on 19-8-2017 by 18thTimeLucky?]
|
|
|
experimental
Harmless

Posts: 21
Registered: 9-7-2017
Member Is Offline
Mood: curious
|
|
That is an impressive crystal, well done 18thTimeLucky?
In my case I'm just purifying some technical grade copper sulfate that contained a lot of impurities. The sulphuric acid solution got the job done,
but the next time I'll try dissolving it in methanol.
|
|
|
18thTimeLucky
Hazard to Self
 
Posts: 51
Registered: 19-8-2017
Location: The one-and-only tea and crumpet land (UK)
Member Is Offline
Mood: 0 Kelvin and still won't crystallise from solution
|
|
Quote: Originally posted by experimental  | That is an impressive crystal, well done 18thTimeLucky?
In my case I'm just purifying some technical grade copper sulfate that contained a lot of impurities. The sulphuric acid solution got the job done,
but the next time I'll try dissolving it in methanol. |
Thanks!
Although methanol does seem like a good solvent to use because of the insolubility of possible contaminants, 10.4g/L is a pretty miserable solubility.
Unless you have several litres of methanol and recover the methanol afterwards then it hardly seems worth the bother.
If you only had a few grams or below of copper sulfate you needed to purify for a reaction then maybe it would be worth it.
Also thats good to know the sulfuric acid solution got the job done, I will try that for when I decide to purify a 500g bag of impure 99% copper
sulfate I have been meaning to get round to!
|
|
|
experimental
Harmless

Posts: 21
Registered: 9-7-2017
Member Is Offline
Mood: curious
|
|
Yeah, didn't notice the 10.4g/L figure. Not that "soluble" actually.
As for sulphuric acid, please consider my case was as follows.
The main method for purifyng copper sulfate I've used is recrystallization. However, after doing several small batches and washing the buchner with
HCl to remove the leftover impurities, some HCl remained in the filtrate.
When I decided to recrystallize a second crop from that filtrate to increase the yield, I got crystals of copper chloride mixed with the desired
copper sulfate crystals. This is where sulphuric acid came to the rescue.
|
|
|
| Pages:
1
2
3 |