| Pages:
1
2
3
4
5 |
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
As for propylene glycol being present (1,2 or 1,3?) Will propanal be formed and if so is it dependent on whether 1,2 or 1,3 prop glycol is present?
And while 1,2 prop glycol will form 2,5dimethyl1,4dioxane will 1,3pg form a larger 1,5 dioxane variant?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2825
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
^the reaction of propylene glycol is different, due to the much lower energy barrier for a pinacol rearrangement. Ethylene glycol gives dioxane while
propylene glycol gives propanal. This is because the carbocation forms:
CH3CHOHCH2OH + H+ >> CH3CH+CH2OH
but
CH2OHCH2OH + H+ >XX CH2+CH2OH (does not form)
The formation of a primary carbocation is forbidden, while a secondary carbocation is allowed. Propanal might undergo side reactions or just boil off.
2,5-dimethyldioxane is not a likely product.
1,3-propylene glycol may give 1,5-dioxocane or oxetane, possibly a mixture of both. However, it is not used in antifreeze.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  | ^the reaction of propylene glycol is different, due to the much lower energy barrier for a pinacol rearrangement. Ethylene glycol gives dioxane while
propylene glycol gives propanal. This is because the carbocation forms:
CH3CHOHCH2OH + H+ >> CH3CH+CH2OH
but
CH2OHCH2OH + H+ >XX CH2+CH2OH (does not form)
The formation of a primary carbocation is forbidden, while a secondary carbocation is allowed. Propanal might undergo side reactions or just boil off.
2,5-dimethyldioxane is not a likely product.
1,3-propylene glycol may give 1,5-dioxocane or oxetane, possibly a mixture of both. However, it is not used in antifreeze. |
Nurdrage did a video on 2,5dimethyl1,4dioxane from 1,2pg so it works.
Just for clarity are U saying 1,3pg won't form propanal but 1,2pg will due
to the OH's being next to each other?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2825
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
No, it's because the carbocation is secondary. 1,3-propylene glycol cannot form a secondary carbocation because both of the alcohols are primary. See:
https://twitter.com/dasingleton/status/1115120772132016129
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Your above sentence is a bit too technical for me
Thats why I asked for clarification.could U simplify it
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2825
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Read the twitter thread. Prof. Singleton explains it better than I ever could. With pictures. See also:
https://en.wikipedia.org/wiki/Carbocation#/media/File:CarboC...
|
|
|
TLutman
Harmless

Posts: 26
Registered: 13-10-2019
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  | Sounds like it worked out. 
The other components in Liquid Fire are also corrosion inhibitors. I wonder what the hell is going on with all of these corrosion inhibitors?
|
Tricky question. If these manufacturers would put them in the msds, people like yourself could figure any reactions. Don’t look at me, I only had a
semester of chemistry as a freshman in high school in the early 80s.
|
|
|
TLutman
Harmless

Posts: 26
Registered: 13-10-2019
Member Is Offline
|
|
Tried this again, paying mind to what was said.
First thing, it wasn’t Traveller brand, it is Pride 1000.
I first distilled the ethylene glycol, not trying to squeeze every drop. I discarded all below 195c, and stopped before it was even done.
I used concentrated H₂SO₄, and did a simple distillation. First distillate came over around 92c, and was yellow in color. At 96c, a clear
distillate came over. I proceeded until 104c and stopped.
I poured that into a separatory funnel and pulled the yellow out, around 10ml. I took it outside and put on a paper towel and held a lighter to it,
and it immediately and rapidly lit on fire, similar to what gasoline would.
I then took the clear distillate and proceeded with the NaOH, and just like the last time, it turned a dark red brown, with almost a suspension in it.
I let it stir for a few hours and tried to gravity filter through a couple coffee filters. It removed nothing, but left the filter stained brown
except the last 13mm of the perimeter, which was clear wet. I took it outside and again held a lighter to it. The perimeter lit up as I would expect,
but the brown stained paper wouldn’t burn.
This is basically the same thing that happened my first go with it. I’m going to grab a different brand and try again, not so much that I need more
dioxane, but because I want this to go the way it’s supposed to.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Sounds like the yellow is what you want and the clear is full of aldehyde/acetal...might want to go with HCl instead (Vogel) after salting out with
not-base and extracting.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Sounds like you skipped the second sulfuric acid wash after distillation
|
|
|
ErgoloidMesylate
Banned
 
Posts: 89
Registered: 8-8-2022
Location: Norad
Member Is Offline
Mood: Freedom of thought is priceless - You can't afford it
|
|
Quote: Originally posted by monolithic  | As a follow up to my last post, I tried fractionally distilling the ethylene glycol + H2SO4. This brought the distillation temperature down a bit but
it was still fairly high. In total,
350 ml ethylene glycol + 35 ml 93% H2SO4, stirred 20 mins, simply distilled to yield 349 g crude distillate.
Added 35 ml 93% H2SO4 to distillate, stirred 20 mins, fractionally distilled to collect 87 - 95 C fraction total mass 205 g.
350 ml ethylene glycol + 35 ml 93% H2SO4, stirred 20 mins, fractionally distilled to yield 360 g crude distillate.
Added 35 ml 93% H2SO4 to distillate, stirred 20 mins, fractionally distilled to collect 87 - 95 C fraction total mass 211 g.
Combined both fractions, 416 g with total volume ~400 ml, and added 70 g KOH. Stirred between 1.0 and 1.5 hours.
Physical appearance: some black sludgy solids, lighter clear aqueous layer on bottom, dirty brown-red organic layer on top.
Discarded bottom aqueous layer, ~100 ml, as well as polymerized sludge.
Added another 25 g KOH, stirred for 1.5 hours. Majority of KOH did not dissolve as water was absent.
Physical appearance: undissolved KOH had some sludge on it, no aqueous layer, organic layer deeper brown-red color.
Gravity filtered solution and fractionally distilled to collect fraction 97.5 C - 102.7 C, crystal clear in appearance.
Total yield 272 g with a measured density of 1.02 g/ml @ 25 C (lit. 1.034 g/ml @ 25 C.)
Stored in an amber glass bottle with ~0.05 g BHT (~200 ppm) over 27 g activated 4A sieves (~10% w/w.)
Cleanup of the black sludge from the ethylene glycol + H2SO4 distillation was kind of shitty. Lots of acetone swirling and the stir bar had to be
scraped clean with a razor blade.
[Edited on 5-24-2020 by monolithic] |
Good to see a small cyclic ether can be made quite easily.
Cannula transfer and cannula filtering is used for lysergic synth Compressed air and remove co2
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Pitty that there are 2 threads with the same topics 1,4-dioxane, here another one:
https://www.sciencemadness.org/whisper/viewthread.php?tid=84...
I did not want to do this reaction after seeing the nasty sticky black residue foaming into condenser in youtube, but later I changed my mind. I did
some adjustments and luckily no problems with foaming from the flask into condenser, also stir bar as well glass stayed clean. I did only the
reaction, not the workup yet. My ideas and improvements:
1.
according the 3 MB attachment by DJF90 the amount of H2SO4 should be 4% (but I do not know whether it is vol% or wt%) and not 10 vol% as used
everywhere in youtube videos as well all experiments here in the forum
I performed the reaction by using third of the sulfuric acid, 11 ml 96% H2SO4 (Lach-Ner) for 300 ml of pure commercial ethylene glycol (fichema.cz)
which is 3,7 vol% of the catalyst. If the 4% should not be volume but weight then only about 6 ml would be enough for 300 ml of
ethylene glycol.
Moreover after distilling out some product, I added fresh ethylene glycol to the flask and continued.
I did 4 cycles, the remainder in the flask before adding fresh ethylene glycol was always approx 100 ml or even more. In the last round when I
distilled out more, there stayed about 50 ml of black viscous liquid in the flask which was easy to pour out.
I processed that way approx 900 grams = 800 ml of ethylene glycol in 4 cycles (distilling out 200 ml of product and then adding 200 ml of ethylene
glycol).
The H2SO4 is only catalyst, not reactant...
2.
I used large 1 L flask for the 300 ml liquid with plenty of empty space to be safe and avoid foaming into condenser (the dead volume and distance from
surface of boiling liquid to the level of condenser also reduces droplets carried into condensate by vapor).
3.
I did not distill too far during the last cycle, for the 11 ml of H2SO4 the remainder in the flask was about 50 ml and no foaming / bumping observed.
I used heater with integrated magnetic stirring.
4.
??? maybe ethylene glycol could be dripped from additional funnel during the reaction at the same drop rate as the volume of condensate ???
5.
??? could be 75% or 85% H3PO4 used too ??? as a legal and maybe more available alternative than H2SO4 for home chemistry???
|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 175
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
Something which may be of interest is this Dow Chemical patent from 1985, which involves using diethylene glycol as a starting material, and running
the reaction under reduced pressure - hence, the reaction occurs at a lower temperature, resulting in less foaming and sludge.
I've not tried it myself, so I can't comment on its feasibility.
Attachment: 1,4-dioxane (1985 patent).pdf (511kB)
This file has been downloaded 372 times
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Lionel Spanner, interesting, my friend Bedlasky brought me diethylene glycol which are they producing. But it is not so available to home chemists
as ethylene glycol.
To prevent foaming and sludge: use less H2SO4, only approx. 4 %, it is only a catalyst. Temperature during the
reaction is approx 140-150 C thanks to high boiling point of ethylene glycol and this temperature is similar as used in production of diethylether
where you drip ethanol into 140-145 C hot mixture of conc. H2SO4 and ethanol. Do not distill too far, e.g. in my case for 11 ml of
conc. H2SO4 I let about 50 ml residue in the flask and did not distill further. Use big flask and fill it to 1/4-1/3, not more, e.g.
for 11 ml of H2SO4 + 300 ml of ethylene glycol I used 1 L flask. My ethylene glycol was also pure (commercial) as well H2SO4.
Moreover I managed to perform 4 cycles with the above amount of catalyst so I processed approx. 900 g = 850 ml of ethylene glycol. I collected approx.
800 ml of condensate. Maybe the ethylene glycol could be added from dropping funnel during the distillation.
I added 10 ml of conc. H2SO4 to the 800 ml of condensate and let to stay overnight. After half a day I performed simple distillation - I collected
everything upto 98 C which gave 500 ml of distillate and later I collected 100 ml of distillate in range 99-108 C. I added excess of K2CO3 to that 100
ml fraction and 25 ml of organic upper phase separated which I tested in small flask with inserted thermometer and on boiling the thermometer read 85
C which is close to azeotrope of 1,4 dioxane with water (88 C).
I will treat also that 500 ml fraction with K2CO3 and then KOH and distill on Hempel column (packed with Raschig rings) and variable ratio
distillation head (that's why I did not yet fractionate it more precisive, I will do that as the final step, until now I hydrolyzed
2-methyl-1,3-dioxolane = acetaldehyde ethylene acetal and now I have to remove all water present).
There stayed about 200 ml residue in the distillation flask so that 10 ml of H2SO4 and the rest could be H2O and some ethylene glycol or maybe
3-hydroxybutanal from acid catalyzed aldol condensation of acetaldehyde.
[Edited on 1-9-2022 by Fery]
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
I processed the distillate with K2CO3 . 1,5 H2O and totally 130 g was necessary, only then it did not dissolve anymore (100 g should be enough if used
anhydrous K2CO3). I kept upper organic layer circa 400-450 ml and I distilled the bottom aqueous layer circa 150-200 ml from which I collected
fraction in range 85-98 C of volume 15 ml of distillate which after salting out with K2CO3 gave 9 ml of dioxane which was added to the main portion.
To the 400-450 + 9 ml ml was added 10 g freshly fused anhydrous K2CO3 (heated in nickel melting pot for 30 minutes at 300 C) which clumped immediately
so then KOH was added incrementally, totally 25 g of KOH was necessary and was let to stay for 2 days at room temperature. Then upper layer separated
(circa 350-400 ml) and bottom layer discarded (circa 50 ml). The upper layer was then let to stay with fresh solid 10 g KOH for half a day, no more
water separated, so the dioxane was then distilled, collected fraction 95-101 C which weighed 350 g and the remainders from distillation (forerun +
residuum in flask) had 20 ml.
I did not dry it with Na as I do not plane any experiment with dioxane soon. It will be dried and redistilled before possible experiment in the
future.
I processed various remainders to increase yield as much as possible. I got 350 g of 1,4 dioxane (not completely dry) from
900 g of ethylene glycol.
|
|
|
TLutman
Harmless

Posts: 26
Registered: 13-10-2019
Member Is Offline
|
|
After little hiatus with other projects, I’m back to this
I tried running the same antifreeze to finish off the gallon to tidy up some shelf space, but tried paying more mind to what was going on.
Last night I distilled the E.G. from the antifreeze (simple distillation). I got my first drips around 100C. No surprises there. It does say it has
added water, but it is concentrated and not premix.
@100C, distillate was cloudy, but uncolored.
@ 150C a slight yellow color was observed, and collection was continued in same flask
Discarded below 170C, approx 150ml
170-180C collected in test tube. Increasing yellow tint- approx 5ml
180C -190C collected in test tube. Yellow tint most noticeable approx 10ml
190-197C new test tube, tint decreasing approx 8ml
Started collecting at 197.1C a pretty clear product at fair rate- 400ml
Interesting was the flask I discarded had a yellow powdery film on the glass when it dried.
I intend to distill crude 1,4 dioxane tonight and believe the yellow layer I initially had will be there. I intend to sep funnel as soon as I finish,
but am thinking about taking different fractions to see when it is coming over.
I’m not seeing others having this issue, and most look like they are collecting right off the bat and yet still have a clear distillate. I don’t
particularly think I’m going to get an answer from anyone because it might be the dye or inhibitors creating the problem and they don’t divulge
those compounds. This is mostly for anyone else who might run into this.
|
|
|
Rainwater
National Hazard
   
Posts: 987
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
From my lab notes a year ago.
https://www.autozone.com/antifreeze-radiator-additives-and-w...
Sds sheet
https://www.autozone.com/batteries-starting-and-charging/bat...
%30 sulfuric acid
In a 3-necked 2 liter rbf roughly 500ml 95% ethylene glycol was added along with 200ml 30% battery grade sulfuric acid.
A stir bar, 5 boiling stones(2mm), 6in thermowell, an addition funnel, and a fractional column were added. The column was insulated with foam.
Atop the column a 3 way distillation adapter with a thermowell w/thermocouple, liebig condenser and 2L receiving flask chilled with ice water
Cooling water was maintained at 25c 2L per minute.
A heating mantle was placed under the boiling flask and a thermometer into its well.
600 The mantle was set to 102c. Stirring was started.
640 After the boiling flask reached 100c, distillation began. Distillate 100c 6 drops/minute
700 boiling flask reached 100c. Distillate 100c
distillation rate increased to the point of overloading the condenser. constant drip & steam
710 Boiling flask reached 102c. distillate is coming over very slowly. 3 drops / minute
Heating set to 130c, first fraction recovered.
730 boiling flask reached 130c. Distillate 103c 30 drops / minute
1100 addition funnel is filled with about 500ml %95 ethylene glycol and slowly dripped into the boiling flask.
1500 addition funnel is filled with about 200ml %95 ethylene glycol and slowly dripped into the boiling flask.
1900 distillation rate has slowed. Distillate 105c 10drops / minute. Tar has began to form in the boiling flask. About 1100ml of distillate has
collected and is placed into the freezer.
The apparatus is turned off for the day.
"You can't do that" - challenge accepted
|
|
|
TLutman
Harmless

Posts: 26
Registered: 13-10-2019
Member Is Offline
|
|
It may very well be this antifreeze brand.
Distilling the E.G. from the antifreeze left varying amounts of a uniform yellow tint all the way to 195C, and probably traces after that.
I had two major fractions in the end from two separate runs. The lower from 180-195 and the 195 up.
I put the lower one of 425ml and 50ml of drain cleaner in a 1000 ml rbf and ran a simple distillation. At 100C I was getting drops in the condenser,
cloudy at first and then started two layers. I could see the drips flowing down the condenser looking odd. I believe the 2 immiscible layers were
coming on the same drop.
I put a test tube under the vac adapter at 100, 114, and 120C and had a denser layer of clear liquid on bottom and a layer of yellow on top. Shaking
showed a quick separation. The yellow was more intense, and is opaque, almost as if all the color in the original EG was pushed out of the crude
dioxane to form a separate layer.
I ran the distillate through a sep funnel a few times through the distillation the rid it of the yellow. I might see if my uv light will light it
up,but it may be the wrong wavelength. I was thinking maybe some sort of uv dye, but this is the cheap antifreeze from the country store up the road.
Not sure if this brand would go so far as to add one.
Maybe tomorrow. Right now is getting everything corked up because that stuff stinks and I don’t want to leave my fumehood run until tomorrow
night.
Rainwater, sounds like you kept some good notes. I could improve on mine a bit. I like your temp and flow on cooling,and that is on my to do list I
wrote up yesterday. Adding a vac guage and means of adjusting is as well.
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
I let the bottle with the product in a fridge for few months at +4 C. Most of the 350 g solidified and there is only circa 20 ml of liquid at the top.
As temperature oscillates slightly around the +4 C the crystals of dioxane repeatedly partially dissolve in places where there are in contact with
liquid and then crystallize back. The density of solid dioxane is higher than the density of solution dioxane+water so after long time all the liquid
mixture of water+dioxane is on the top of a solid block of pure dioxane crystals and no liquid is trapped among solid dioxane so all the liquid
containing water could be easy poured out. Even that 20 ml of liquid should be mostly dioxane and only a little of water.
[Edited on 15-11-2022 by Fery]
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Well done guys!
TLutman good that you distilled and purified your ethylene glycol, >150 ml of forerun and 400 ml of main fraction is a lot. Bottles with antifreeze
should have a table which tells temperatures at which antifreeze could be used and also its various mixtures with water. A mixture water:glycol 1:3
should have worse temperature usage than concentrated glycol - the dilution of antifreeze could be perhaps guessed from this worse temperature when
reading the table from bottle in a shop.
TLutman and Rainwater - why do you use such amount of H2SO4? It is only a catalyst. I used <4 vol % and the etherification reaction ran well. I
repeatedly added fresh ethylene glycol into the remainder (4 distillation cycles) and still not overrun at all unlike seen in videos on youtube,
remainder darkened in color into black but was easy to pour out after the last cycle and clean the flask with just water.
https://www.sciencemadness.org/whisper/viewthread.php?tid=65...
Rainwater you used stirbar with boiling stones - did it bring some advantages? By my experience the stirring is necessary but not boiling stones then.
You put all 1100 ml of distillate into freezer - what was the result? Wasn't it contaminated with acetaldehyde? Anyway you cooling water temperature
was above its b.p. but b.p. of another sideproduct 2-methyl 1,3-dioxolane is 83 C. I guess your distillate contains still this sideproduct and also a
lot of water as it should be an azeotrope dioxane:water. I got rid of 2-methyl 1,3-dioxolane by a hydrolysis using a little of H2SO4, there was enough
water in the azeotrope to hydrolyze it. Then I distilled it and obtained azeotrope dioxane:water but now without 2-methyl 1,3-dioxolane. Then I dried
it using K2CO3 and KOH and distilled it again. This product was then placed into fridge at + 4 C for few months. Putting into freezer (-18 C) would
freeze not only dioxane but also the water remained after drying the product. So I used only fridge +4 C and not freezer -18 C.
|
|
|
Rainwater
National Hazard
   
Posts: 987
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
| Quote: |
why do you use such amount of H2SO4?
|
My acid was very diluted. All the readings on this procedure suggested 40~60ml of 99% Sulfuric acid
the molar equivalent is 133~200ml of 30% consentrated.
| Quote: |
Rainwater you used stirbar with boiling stones - did it bring some advantages? |
The stir bar slings the stones around the flask and i think that helps stop bumping, but my heating mantle loves to decouple from the stirbar and when
its not spinning properly the stones help me hear there is a problem. They are quite loud when everything is just right.
| Quote: | | You put all 1100 ml of distillate into freezer - what was the result? |
A yellow semi-solid mass. It stopped most of the reactions and allowed me to store the crude product safely stoppered until i could work on it again.
I should note that the flask still build up pressure
| Quote: | | Wasn't it contaminated with acetaldehyde? |
yep.
I purified it a few days later. Fractional distillation in acidified conditions, koh soaking, decant. Then used it to clean crude sodium metal which
also served to further purify the dioxane.
Waste disposal i dont remember how much i got below 80c during the second distilation, something l8ke 150~200ml i think. I suck at keeping track
of yields.
| Quote: | | Putting into freezer (-18 C) would freeze not only dioxane but also the water remained after drying the product. So I used only fridge +4 C and not
freezer -18 C. |
Very true, I figured that my rbf was almost half way full, so if the water did freeze solid it wouldnt break my flask, and my freezer is set to -5c
but from the readings i didnt expect it to freeze solid
( digging. Please wait )
https://www.journal.csj.jp/doi/abs/10.1246/bcsj.46.2965
Attachment: bcsj.46.2965.pdf (692kB)
This file has been downloaded 326 times
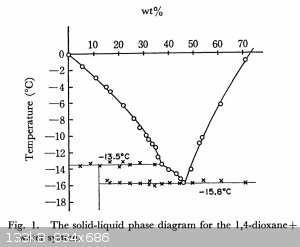
1,4 dioxane + water melting points
"You can't do that" - challenge accepted
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Rainwater - well done. Luckily dioxane on solidification does NOT expand in volume unlike water. It is possible to remove most of the water still
present after KOH drying and then distillation - by solidifying the almost dry dioxane in a fridge at +4 C for (few months better than only few days)
and pouring out small amount of liquid on top of solid mass of dioxane. Then you need only tiny amount of hard to get sodium metal to dry it
completely.
Guys I like you are doing experiments. The more experiments, the better!
|
|
|
TLutman
Harmless

Posts: 26
Registered: 13-10-2019
Member Is Offline
|
|
After some additional digging I found this is formulated for gas and Diesel engines. It has a few more components not on the bottle.
Boron >200ppm
Nitrite/moly >1600ppm
Inhibitors>2%
Glycol 95-97%
Not sure if the inhibitors add anything different than normal for the diesel application other than low silicates.
I ran the second distillation with an additional 10ml H2SO4, collecting from 84-94C using a fractional column. I don’t have a total on the
distillate yet as it’s stirring with some KOH, but it is depressing in both amount and that it is a uniform red with no separation yet.
|
|
|
Rainwater
National Hazard
   
Posts: 987
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
If you are not getting separation, add more Koh. Keep adding in small amounts until some of the hydroxide does not dissolve.
"You can't do that" - challenge accepted
|
|
|
TLutman
Harmless

Posts: 26
Registered: 13-10-2019
Member Is Offline
|
|
I added more koh earlier, and it went from red to black. All koh dissolved, but the returns on the effort and amount of koh used has me ready to set
aside this brand antifreeze and try another to see if it’s me or the antifreeze. I’m estimating out of 350ml of crude product, I got under 100ml
from 84-94C, and with the losses from the koh , yield will be truly horrible.
I am happy I never got a sludge volcano, but I do have some black slime/tar that is going to be fun.
|
|
|
| Pages:
1
2
3
4
5 |