| Pages:
1
2
3
4 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Basically i'd like to try to fabricobble a very amateur Gas Chromatograph some day.
A FID will need hydrogen, so NaOH + Al seems to make that OK.
Hydrogen might be OK for the carrier gas, just that it'd need much less volume of Nitrogen, based on what i've managed to find in literature.
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Distilling under N2 is very doable. I've never done it with generated gas, only from a tank, but it should work much the same.
Sparge the cold distillate before turning on the heat, you want to drive off dissolved gases in the solvent. When the heat starts to rise, switch to a
blanket, applying the gas under positive pressure from the top of the apparatus. The blanket doesn't need to actually have a gas flow, just a positive
pressure. Add a bubbler at the end of the gas generating train, so the system isn't closed.
Back in grad school days, we did this exact kind of thing, with a tank feeding a drying tube, then a hose manifold, with the bubbler at the end. The
bubbler had a little ball in it, making a slow constant ticking sound, so you knew when the gas was flowing and at about what rate. (Fast for sparge,
slow for blanket). Hoses leading to the apparatus were terminated with Luer lock needles, and balloons of N2 (yes, actual kids toy balloons) also
fitted with syringe needles could also be applied to ensure pressure.
Reagents could be transferred with syringes, or siphoned under pressure using a cannula. Apparatus was sealed with septa.
We could do most of what would otherwise require Schlenkware, and at a far lower cost. Usually faster, too.
We ran solvent stills in much the same way, while there was a departmental ether still in an unused lab, in its own hood, behind a blast shield, our
group distilled our own DCM, THF, and dimethoxyethane.
[Edited on 1/23/18 by PirateDocBrown]
Phlogiston manufacturer/supplier.
For all your phlogiston needs.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Yeah, an oxygen concentrator will separate nitrogen and oxygen. Not perfectly, so you may need a scrubber if the goal is to get it really pure. The
contents of those chemical hand warmers are a good oxygen scavenger. They're mostly reduced iron, with some salt and water as catalysts, among other
things. I believe CO2 behaves more like O2 in this system, but I'm not 100% sure.
I've wanted an oxygen concentrator for a while now. It'd allow efficient ozonolysis, hotter flames, improved catalytic oxidation, and other fun
stuff. It'd be good for a lot of things other than as a source of nitrogen.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
BTW, if you have nasty deposits and stains on your glassware left by oxidizing iron on it, they come right off when you soak it in full strength
muriatic acid. Of course, it might be cheaper to just replace the glass....
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I'm interested in generating pure nitrogen for my next element video, so it's good to see this topic resurface.
My plan was to use sodium nitrite and ammonium chloride:
NH<sub>4</sub>Cl(aq) + NaNO<sub>2</sub>(aq) → N<sub>2</sub>(g) + NaCl(aq) + 2 H<sub>2</sub>O (l)
JJay's mention of a "pinhole purification" method is very interesting. This led me down a rabbit hole of researching Graham's Law,
then effusion, then mean free path, etc. A cursory look tells me that the pinhole needs to be smaller than the mean free path of air molecules to
enable separation by effusion, and the mean free path for air at ambient pressure is 68nm. I guess you'd have to find a porous membrane with that pore size, then, since this is orders of magnitude smaller than any hole I can
physically poke. This approach might be out of reach of the amateur experimenter. You might have to set up multiple diffusion chambers, like a uranium
purification plant! Interesting idea though; I'll be sure to mention it.
[Edited on 1-24-2018 by MrHomeScientist]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
The nitrogen isn't going to be completely pure after a single pass through a pinhole, even a 68 nm membrane. If you wanted 100% separation with a
single pass through a pinhole, the pinhole would have to be smaller than the minimum free path of one of the gases; it would be essentially just
filtering. Using pinholes (or 68 nm membranes) is more like chromatography.
It's certainly not out of the reach of the amateur experimenter, but it's probably not something an amateur would use to produce useful quantities of
nitrogen gas, even with a 68 nm membrane. I was mainly joking about the idea of producing "enriched nitrogen" by gas effusion and was surprised to see
it attacked so vigorously, but it's an idea that is easy to defend....
[Edited on 24-1-2018 by JJay]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Could someone in the USA please give these guys a call and find out about prices :
Membrane sales: +1.925.431.1030
HQ : +1.713.937.5200
https://www.generon.com/product/nitrogen-membrane-separators...
From their website :
"By simply supplying the GENERON® membrane
modules with compressed air, they will generate a
nitrogen stream"
If they are cheap enough, that'd be an attractive option.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
They're using a 10nm membrane... that's small enough to effect a separation significantly greater than a Graham's law pinhole.
Generon deals with industry in countries all over the planet - why not just drop them an email message?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I don't speak American well enough.
The last time i tried, they guy said "i'm travelling" so i reasoned that he was on a train or 'plane (i.e. not busy) and asked him a load more. He got
annoyed and told me to f**k off.
When i asked a local natural American what the term "travelling" meant to them, it turned out that it translates to "out of the office and generally
unavailable to answer questions".
I didn't know that at the time.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I sent Generon a request for information on their membrane filters and how the technology works. That's incredible that you can filter gases straight
out of compressed air.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | I don't speak American well enough.
The last time i tried, they guy said "i'm travelling" so i reasoned that he was on a train or 'plane (i.e. not busy) and asked him a load more. He got
annoyed and told me to f**k off.
When i asked a local natural American what the term "travelling" meant to them, it turned out that it translates to "out of the office and generally
unavailable to answer questions".
I didn't know that at the time. |
At least you wernt smoking Fags! and complaining about having a sore fanny. 
But I do think it helps to have a native do the introduction first, otherwise you got to explain why you havnt been to tea with Liz.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Thanks MrHomeScientist.
I still find it hard to believe that filtering Air actually works.
Please update us on what they tell you.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Yeah, I've looked this up before. Trouble is, you still need two outlets, otherwise the unfiltered gas will just build up pressure. Oh, and did I
mention that they're ridiculously expensive? I probably should have mentioned that part first. Thousands of dollars for the minimum-price one.
Apparently, there are two types, one that uses actual filters, and one that uses columns packed with microfibers. For the microfibers, one of the
gases tends to stick to the fibers more than the other, which slows that gas down. So as they move horizontally in the column, they tend to stratify
vertically, and can be collected via outlets on the column.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
It's also possible to fractionally distill liquid air.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by vmelkon  | Probably the cheapest way is to use iron + water. It will also react with the oxygen in the air and make Fe(OH)2. The left over gas will be mostly N2
and argon.
You can also heat some metal powder to react with the oxygen. |
Quote: Originally posted by Melgar  | Yeah, an oxygen concentrator will separate nitrogen and oxygen. Not perfectly, so you may need a scrubber if the goal is to get it really pure. The
contents of those chemical hand warmers are a good oxygen scavenger. They're mostly reduced iron, with some salt and water as catalysts, among other
things. I believe CO2 behaves more like O2 in this system, but I'm not 100% sure.
....... |
I remember a report of workers in a large iron pipe that did not fair well when water was drained exposing a fresh iron surface which started an
electrochemical reaction that consumed all the available oxygen. Placing a burning split in a vessel also removes O2 rapidly leaving N2 with some CO
and CO2.
Combines the paths by adding a mix of salt, water and fresh iron powder to a large squeezable bag/bottle, hold a lit split until it extinguishes,
close vessel fitted with an exit tube and shake to dissolve CO2 gas. Let stand overnight. Compress vessel to produce a stream of N2 and impurities
that are passed into a hot washing solution of say Na2CO3.
Low tech with safe, available and inexpensive reagents, but may have some CO impurity in the nitrogen gas. The carbon monoxide, if a problematic
presence, can be removed with FeI2 via the reaction:
FeI2 + 4 CO → Fe(CO)4I2
[Edited on 29-1-2018 by AJKOER]
|
|
|
Pumukli
National Hazard
   
Posts: 705
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
A year ago I systematically hunted "oldies but goldies" - looking articles from JACS. You can find some really interesting (not to mention: fairly
amateur friendly) articles which were cutting edge science a century ago. Now you can do them in the garage.
Anyway, among others, I saved the following one:
A Nitrogen generator
by Chas Van Brunt, received May 4, 1914.
from the research laboratory of General Electric Company, Schenectady, New York.
Enjoy!
Attachment: nitrogengenerator.pdf (187kB)
This file has been downloaded 555 times
edited typo
[Edited on 29-1-2018 by Pumukli]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Awesome Pumukli !
Many thanks for that Gem.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I'm emailing with a guy at Generon; they offered to send me a free module to demo in a video! I'm going to ask about what their requirements are for
using their product in my video. I'm worried they'll want to restrict what I can and can't say about it, and I don't want this to turn into like a
product review where I can only say positive things or something.
In the meantime, here's a patent for the filtration system that explains it pretty well: https://www.google.com/patents/US7517388 (also attached below for posterity)
Turns out, the guy I'm talking to is the inventor!
Attachment: US7517388.pdf (883kB)
This file has been downloaded 424 times
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
WTF ?!?!?!
Superb stuff MrHomeScientist !
Fantastic that they are sending you the Real Deal, and for Free !
Being in touch with the inventor is, well, AWESOME ! I guess any questions you have can be answered.
Now, You may be getting one for free, but is there any indication of how much they normally sell for ?
You definitely have to make that video. Most definitely.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
In one of the patents cited by the one I linked above, US 9034957, it lists the type of polymer used in the membrane fibers:
| Quote: | | The composition is a spin dope including tetrabromo bis-phenol A polycarbonate (TBBA-PC) and tetrabromo bishydroxyphenylfluorene polycarbonate
(TBBHPF-PC), in proportions, by weight, ranging (in percent) from about 60/40 to 40/60, and n-methyl pyrrolidinone (NMP) and triethylene glycol (TEG),
wherein the ratio of the amounts of NMP to TEG, by weight, is in the range of about 1.6-2.5. The spin dope is used to make hollow fibers for use in
gas-separation membrane modules. |
For those that didn't read the patent, the "filter" consists of a bundle of hollow polymer fibers that pressurized air is passed through. Different
gases permeate through the membrane at different rates, so oxygen and water vapor pass through the walls of the fibers while nitrogen continues down
the tubes. Nitrogen is collected from the bottom of the fibers while oxygen is drawn off from the space surrounding the fibers. That also suggests
that enriched oxygen gas can also be obtained from such a filter.
According to Wikipedia, it works because there is a chemical potential between the inside and outside of the fibers caused by the pressure difference. So
oxygen passes through the membrane faster than nitrogen, and the pressure in the system drives the nitrogen out the bottom before it can also diffuse
out.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
It has arrived!

I got the tubing and Swagelok fittings from McMaster at the same time. Now, I'll order the compressed air tank from AirGas and I should be good to go.
Here's the plan for the setup:
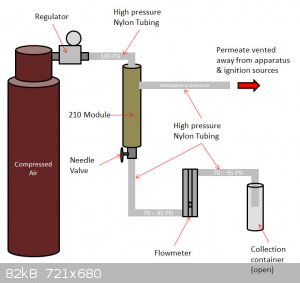
The permeate is the filtered oxygen, at up to 50% concentration, and the retentate is pure nitrogen, collected in an open flask at the end. I included
a flowmeter (rotameter) to help gauge the purity; the spec sheet for the 210 module relates purity to output flow rate. Slightly concerning that I'll
be pushing the rotameter close to its max 100 PSI rating, but here's hoping nothing explodes!
I have one month to use the filter before I need to return it to Generon, so the pressure is on! Pun intended!
|
|
|
sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
good evening
I'm grateful for so much information. but I have a doubt, after purifying nitrogen, how do I fix nitrogen, is there any substance that can react with
nitrogen by making a nitride, I'm using iron oxide(I saw in wikipedia that iron oxide is a photoanode when exposed to the sun?), along with magnesium
oxide inside a copper tube and a heated candle, does it work?
I saw in the previous comments that iron and water works, but I live in a forest, far from the city, and still can not make a tool to extract the iron
from the minerals, I can only extract the iron oxide with caustic soda and peroxide of hydrogen
[Edited on 20-2-2018 by sclarenonz]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Nitrogen is an active area of modern chemical research because it's incredibly difficult. You could use the Ostwald process, with terrible efficiency,
or you can absorb the nitrogen onto metallic lithium. That's about it; there are some very complex catalysts which fix nitrogen, some of which you
might be able to buy, none of which are cheap... Or fast.
|
|
|
BaFuxa
Hazard to Self
 
Posts: 61
Registered: 18-9-2017
Location: Mars
Member Is Offline
Mood: Buzzing
|
|
Quote: Originally posted by sclarenonz  | good evening
I'm grateful for so much information. but I have a doubt, after purifying nitrogen, how do I fix nitrogen, is there any substance that can react with
nitrogen by making a nitride, I'm using iron oxide(I saw in wikipedia that iron oxide is a photoanode when exposed to the sun?), along with magnesium
oxide inside a copper tube and a heated candle, does it work?
I saw in the previous comments that iron and water works, but I live in a forest, far from the city, and still can not make a tool to extract the iron
from the minerals, I can only extract the iron oxide with caustic soda and peroxide of hydrogen
[Edited on 20-2-2018 by sclarenonz] |
Magnesium nitride works. See the Serpek process.
Potential counts for nothing until realized.
|
|
|
sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
Good day
I found this video interesting.
https://www.youtube.com/watch?v=ZRFai58q1J8
because of the inverse effect
MgO + NH3 = Mg + N2 + H2O
Al + MgO = Al2O3 + Mg
if magnesium is a great fixative of nitrogen, I am now looking to isolate magnesium, and I saw that it is possible with ammonia, is it possible?
[Edited on 24-2-2018 by sclarenonz]
[Edited on 24-2-2018 by sclarenonz]
|
|
|
| Pages:
1
2
3
4 |