| Pages:
1
2
3 |
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by cmos6667  |
Before you get any wrong idea: the alkene is at the end position, so wacker or performic or whatever won't work, you need a catalyst under inert
atmosphere to rearrange it to the middle  |
actually,wacker oxidation will convert terminal olefins to methyl ketones,so it will work very well.And you don't need any special catalyst or inert
atmosphere,conc KOH with heat will do the job
for the dehydration,why can't phosphoric acid be used?
85% phosphoric acid is available as rust remover
also,instead of using cinnamaldehyde,why can't cinnamic acid be used? one could cyclopropanate the double bond(if choroform is used,then you will get
dichlorocarbene instead of methylene group which won't make much of a difference, I think,and if diazomethane is used,then the COOH should be
esterified first),reduce it (catalytic hydrogenation or maybe even Zn/AcOH)to get 2-methyl-3-phenylpropanoic acid and then do schimdt reaction to get
the amphetamine.
[Edited on 20-5-2015 by CuReUS]

|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
That's a rather tedious process, no?
Use nitric acid and iron wool and put cinnamaldehyde on acidic alumina --> get carboxylic acid nitrostyrene, then use P + I2 followed by lots of
NaBH4, and again P + I2 followed by Mg
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Will the HI (P + I2, [ as cited in March]) reduction work on reduction of beta nitrostyrene to the corresponding ketone? I doubt it but maybe I'm (as
often) wrong.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Alcohols and aldehydes like cinnamyl alcohol and cinnamaldehyde going into reductions to alkanes (or alkenes) with Clemensen reduction (Zn/Hg + HCL)
using diethyl ether as a solvent, gassing HCL trought it at a temperature of - 15 º C, like zgoat65 said:
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6...
Both are sold as perfumery flagrancies, at least here, in Latin America.
It will produce a mix of propenyl benzene and allyl benzene, and the last can be isomerised to the first by heating with KOH. Propenyl benzene trated
with performic acid leads to 2 phenyl propanone as you can see in the attachment below.
Phenyl Propanone forms amphetamine or methamphetamine by Leuckart Reaction with formic acid and ammonia or methylamine (formamide or n-methyl
formamide as well), reaction which were already discussed in another topics here in this forum.
Here is an interesting Methamphetamine You tube Leuckart reaction video:
https://www.youtube.com/watch?v=nAIlFwaBju0
Attachment: performic-oxidation-of-safrole and phenyl propene (further isomerization).pdf (1MB)
This file has been downloaded 828 times
[Edited on 8-5-2016 by Chemi Pharma]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I'm pretty sure that any dissolving metal reduction, including Zn/HCl, will saturate the C=C bond in cinnamaldehyde, giving phenylpropanol or
phenylpropane.
Hydrazine on the other hand may be selective enough to work.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
It seems that the double bond presented in cinnamyl alcohol and cinnamaldehyde is quite resistent to reduction. I reproduce below a text published at
rhodium pages, where a guy called "Psychokitty" experiments this kind of reduction with cinnamyl alcohol affording 60% yield of allylbenzene (4 parts)
and propenylbenzene (1 part). He broght as reference Tetrahedron 27, 5081 (1971).
Another guy called "Rev Drone" claims even LIALH4 can't break the double bond afther chlorination with mesyl choride and further reduction, giving a
lot of references and discussing others routes.
Here's the text:
"Psychokitty:
This appears optional. According to the review, commercial Zn dust worked just fine when used in procedure A.
Commercial zinc dust (16 g., 325 mesh) was activated by stirring for 3-4 minutes with 100 ml of 2% hydrochloric acid. The zinc was immediately
filtered under suction, washed to neutrality with water, and then washed with 50 ml of ethanol, 100 ml of acetone, and diethyl ether. The resulting
powder was dried at 90°C under vacuum (10 minutes) and was used within 10 hours of preparation.
Procedure A:
Cinnamyl alcohol (1.30 mm) was dissolved in 75 ml of dry ether saturated with hydrogen chloride at 0°C. Activated zinc dust (5.0 g; 0.076 mol) was
slowly added to the cooled mixture with vigorous stirring at a rate such that the temperature maintained below 5°C. The reaction was exothermic and
considerable hydrogen evolution occurred. The reaction mixture was stirred for 1 hour at 0°C and then filtered. The filtrate was shaken with 500 ml
of ice water and then washed to neutrality with aqueous sodium carbonate. The aqueous washings were dried over sodium sulfate and evaporated under
vacuum. Chromotography of the residual oil over silica gel (Mallinckrodt, 100 mesh, 25 g) using benzene as eluant afforded 60% yield of allylbenzene
(4 parts) and propenylbenzene (1 part).
The above ratios seems a little steep for the average bee with the volume of solvent too great. A more practical method that is on a preparative scale
can be found in Tetrahedron 27, 5081 (1971).
Rev Drone:
I know nobody likes it, but how about going from cinnamyl alcohol to allyl benzene using LAH? Yeah, its overkill, and yeah its water sensitivity is a
pain-in-the-ass, but you know it'll get the job done. The work-up should be particularily easy, as far as LAH reduction work-ups go around here. A
little esterification with mesyl chloride wouldn't hurt things either (but isn't necessary unless you're really worried about maximizing your yield.)
Ref's:
• Indian J. Chem. Sect. B 23(4), 303-306 (1984)
• Carbohydr. Res. 141, 49-56 (1985)
• Liebigs Ann. Chem. 12, 1249-1255 (1990)
• Liebigs Ann. Chem. 1, 87-94 (1992)
• J. Org. Chem. 60, 872-882 (1995)
Also, you could go this route:
cinnamyl alcohol + HI/RP -> propenyl benzene
( "" -> phenylacetone)
Ref = Chem.Ber. 11, 671 (1878)
Just to add onto Rhodium's idea of cinnamyl alcohol -> 3-phenyl-1-pronanol -> allyl benzene:
aryl propenyl alcohol + H2 + (Cat.) -> 3-aryl-1-propanol
• (Cat.) = Pd/SrCO3
• J. Chem. Soc. 618, 619 (1953)
• (Cat.) = Pd
• Justus Liebigs Ann. Chem. 401, 151 (1913) [Anm.]
• J. Org. Chem. 24, 736, 740 (1959)
• Phytochemistry 20(6), 1543-1546 (1981)
• (Cat.) = nickel
• Chem. Zentralbl. 95(I), 1878 (1924)
• Zh. Obshch. Khim. 20, 1199, 1206 (1950)
• Acta Chem. Scand. 15, 357-369 (1961)
•
• (Cat.) = Ni2B (nickel boride)
• Chem. Pharm. Bull. 38(6), 1720-1723 (1990)
There are actually dozens more ref's on this, but I'm getting tired of listing them, so I think you get the general idea... Anyways, the next question
is: what about the ref's for the dehydration? The "cinnamyl alcohol + -> allyl benzene" step? Well,
3-Phenyl-1-Propanol --[Reagent]--> Allyl benzene
• SOCl2
• C.R.Hebd.Seances Acad. Sci. 188, 638 (1929)
• Ac2O
• JACS 78, 584, 589 (1956)
• Helv. Chim. Acta 62, 135-139 (1979)
• H3PO4
• JACS 57, 151, 155 (1935)
• Mol. Sieves
• J. Chem. Soc. Faraday Trans. 1, 78, 2017-2022 (1982)
Cinnamyl alcohol to propenylbenzene
This contradictory response was started last night, before it became contradictory.
There are numerous literature references for the SN2 substitution of the OH in cinnamyl alcohol for chloride, bromide and iodide, using HCl, HBr and
HI respectively. In fact, HI can go on to reduce the alkyl iodide to give the ever-usable propenylbenzene in one-pot.
The substitution works probably because of the conjugation of the molecule, which gives a greatly stabilised transition state for the SN2 displacement
(much like the rate enhancing effects when a leaving group is alpha- to a ketone). The addition across the double bond happens at its usual (slower)
rate; addition across the double bond also disrupts the favourable conjugation. Because of these effects, the -OH of cinnamyl alcohol behaves
effectively like the reactive -OH of benzyl alcohol.
I would suggest adding HBr to cinnamyl alcohol to give cinnamyl bromide, followed by reduction to propenylbenzene with borohydride/PTC. Many
reductions give a mixture of products (e.g. allylbenzene/propenylbenzene/propylbenzene) but with a suitable hydride nucleophile you can selectively
displace the bromide with hydride (again, this may be made easier because of the conjugation stabilising the transition state).
Here are some references (some of which are available online for free). Some of them look very interesting:
Cinnamyl chloride from cinnamyl alcohol with HCl
J.Amer.Chem.Soc. 107 (7), 1985, 2033-2046
Chem.Ber., 39, 1906, 2553
Arch.Pharm.(Weinheim Ger.), 247, 1909, 349
J.Org.Chem., 42, 1977, 871-875
Justus Liebigs Ann. Chem., 479, 1930, 211, 248
J.Chem.Soc., 1941, 507, 510.
Cinnamyl chloride from cinnamyl alcohol using other sources of chloride
SOCl2, 94% yield: Org.Lett., 5 (8), 2003, 1167-1170
PPh3/trichlorocyanuric acid: Synth.Commun., 32 (17) 2002, 2691-2694
Trichlorocyanuric acid/DMF/DCM, 92% yield: Org.Lett. 4 (4), 2002, 553-556
SOCl2/benzotriazole, 100% yield: Syn.Lett., 11, 1999, 1763-1765
Potassium carbonate/chlorotrimethylsilane, 91% yield: Synthesis, 4, 1983, 314-315 [Article in German].
Cinnamyl bromide from cinnamyl alcohol with HBr
J.Amer.Chem.Soc. 38, 1916, 1076
Chem.Ber., 58, 1925, 280
J.Org.Chem., 25, 1960, 1719-1722
J.Chem.Soc., 97, 1910, 426
Chem.Ber. 39, 1906, 2553.
Cinnamyl bromide from cinnamyl alcohol using other sources of bromide
PBr3: Chem.Ber. 43, 1910, 178
NaBr/BF3/acetonitrile (79% yield): Tetrahedron Lett., 26 (32), 1985, 3863-3866
Hexamethyldisilane/pyridinium bromide perbromide (100% yield): J.Org.Chem. 45 (9), 1980, 1638-1639
Chlorotrimethylsilane/lithium bromide (93% yield): J.Org.Chem., 45 (9), 1980, 1638-1639.
Cinnamyl iodide from cinnamyl alcohol with HI
Justus Liebigs Ann. Chem. 479, 1930, 211, 248.
Cinnamyl alcohol to propenylbenzene with HI
Chem.Ber., 11, 1878, 671.
Reduction of cinnamyl bromide to propenylbenzene
NaBH4/PTC, 80% yield: J.Org.Chem., 46 (19), 1981, 3909-3911
Lithium tri-sec butyl borohydride, 99% yield: Bull.Chem.Soc.Jpn., 58 (2), 1985, 789-790
Zn(BH4)2, 60% yield: Angew.Chem., 95 (7), 1983, 568-569 [Article in German]
LiAlH(i-Bu)2(n-Bu), 95% yield: J.Org.Chem. 49 (10), 1984, 1717-1724.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Generalizing the reactivity of cinnamyl alcohol to cinnamaldehyde is a fool's errand. Most reductions of an alpha,beta-unsaturated aldehyde will
attack the C=C bond before the C=O bond. This is because the reaction is really a 1,4 conjugate reduction.
If cinnamaldehyde were reduced to allylbenzene by Zn/HCl -- the cheapest reducing agent around -- someone would have noticed.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Clearly, in organic chemistry reactions not ever the products follow what to be expected. Read the article linked below and you will see that
cinnamaldehyde reduced with LAH can give propenyl benzene, or propyl benzene, according the manner you conduct the reaction. "add LAH to
cinnamaldehyde and you get just reduction of the carbonyl group; invert the order of addition and you additionally get reduction of the double bond",
the researcher claims:
http://www.ch.imperial.ac.uk/rzepa/blog/?p=13688
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
I remember reading somewhere that if a phenyl ring was attached to the b-carbon of an a,b unsaturated carbonyl compound(like cinnamaldehyde),there
would be reduction of the double bond along with reduction of the carbonyl group to alcohol using LiAlH4
[Edited on 8-5-2016 by CuReUS]
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
I apologize. Cinamaldehyde reduction with LAH gives hydrocinnamyl alcohol or phenylpropyl alcohol, nor propenyl benzene or propyl benzene like i
wrote. See:
F.A. Hochstein, and W.G. Brown, "Addition of Lithium Aluminum Hydride to Double Bonds", J. Am. Chem. Soc., vol. 70, pp. 3484-3486, 1948. http://dx.doi.org/10.1021/ja01190a082
But the question is: Why some experiments, like Psychokitty did (as posted above) with activated zinc and HCL reduces the C=O group to alkane, and
don't touch the C=C bond ???
Do Clemensen reduction works with cinnamaldehyde reducing the C=O group to CH2 preserving the C=C bond affording propenyl benzene and allyl benzene
like Cinnamyl alcohol does ???
My Lab is still under construction and i enjoy if someone could test this and post the results here.
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
Whilst I don't think this thread deserves as much attention as it has, I stumbled upon this paper earlier which deals with the exact problem you're
discussing and almost completely OTC: http://www.organic-chemistry.org/abstracts/lit2/441.shtm
Turn your fruity flavored Ethyl cinnamte into Diydrocinnamyl alcohol in just 24 hours.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Sorry Nitrerat but i don't see any relationship between what you have posted and what we are discussing here.
Cinnamyl esther to cinnamyl alcohol could be afforded by too many routes.
What is on discussion here is why cinnamyl alcohol suffers clemensen reduction to propenyl benzene and allyl benzene and cinnamaldehyde doesn't. I
claim that it works, but other members think the C=C bond will be reduced as well, affording propylbenzene.
I wonder if you think about this matter and bring references, pratical results or a solid study that validates or refute this theory.
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
You're right Chemi Pharma, my apologies.
But perhaps I can offer a little help. I imagine the differences in the reductions is because the of all the resonant forms cinnamaldehyde can take
on.
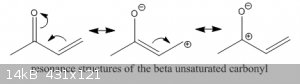
I don't know if a Clemmensen reduction is completely selective of just reducing carbonyls to hydrocarbons. I don't remember the reference but I'm
sure in some cases it can reduce carbonyls to alkenes.
[Edited on 5/10/2016 by NitreRat]
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
According to this paper I found in the Rhodium archives A Modified Clemmensen Reduction Procedure for Conversion of Aryl Ketones Into Aryl Alkenes
| Quote: |
...While this procedure was successful with many compounds, variations were devised in order to widen the applicability of the reduction.2-4 In some
instances the formation of alkenes was observed...
|
I don't have access to any of the papers it references though so this might be completely useless
[Edited on 5/10/2016 by NitreRat]
|
|
|
| Pages:
1
2
3 |
|