| Pages:
1
2
3
4 |
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
Bert,
For the oven, at the time I was thinking 180C on a glass dish would suffice but I now realize that may not be sufficient. I am still of the opinion
that even without drying, NaOH can still be used reliably in a titration.
What do you think about the hydration matter?
With the Acid, my mistake was pointed out in the next post and in the next after that, I acknowledged my mistake.
Maybe you missed that.
I realize now that I spelled 'Hygroscopy' with a 'D' and I apologize for that.
Are you saying however that "The hyGroscopy is minimal" is not a valid sentence? I know for certain that saying "The hydroscopic is minimal" is not
correct. Here is a page on Wikipedia that describes "Hygroscopy" as "the ability of a substance to attract and hold water molecules from the surrounding
environment."
Molecular Manipulations, thankyou for the reference, I will take the time to thoroughly look through it.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Melt a small amount of Sodium hydroxide (318 C if anhydrous, about 250 C @ 10% water) on a glass surface, one which you do not mind losing. Please
report on the condition of the glass afterwards. Draw conclusion on suitability of high temperature/glass containment method for dehydration of this
chemical.
Sodium hydroxide is not only hyGroscopic, but deliquescent. It will take up water from the air until it DISSOLVES itself, and then continue to absorb
even more water... As mentioned by others, it will also scavenge atmospheric CO2 and convert itself to carbonate.
In general, if you have not performed an operation, or had hands on experience with a material- but wish offer advice? Please make it clear you
discuss it from your reading (and give your references!). Or clearly say what you provide is CONJECTURE, rather than authoritatively state a
procedure. Someone who knows even less than you may take your advice as gospel, perhaps with a bad outcome.
[Edited on 18-2-2015 by Bert]
[Edited on 18-2-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
| Quote: | | Sodium hydroxide is not only hyGroscopic, but deliquescent. It will take up water from the air until it DISSOLVES itself, and then continue to absorb
even more water... As mentioned by others, it will also scavenge atmospheric CO2 and convert itself to carbonate. |
What we were discussing was whether NaOH hydrates (ie. integrates water into it's crystal structure).
| Quote: | | Draw conclusion on suitability of high temperature/glass containment method for dehydration of this chemical. |
As I said, if I had to put it in the oven, I would have set the temp 180C, which is below melting point of NaOH.
Sorry if I sound stupid, but I'm not quite sure what that diagram is supposed to mean.
I freely admit there is MOUNTAINS of stuff I still need to learn and would be more than pleased if someone is willing to teach. Meanwhile, I like to
pass on what knowledge I do know to others in this forum where I can be corrected if wrong.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Trizocy
Harmless

Posts: 34
Registered: 16-2-2015
Member Is Offline
Mood: No Mood
|
|
Wow guys, i am really charmed with all the help i got 
Can say this, was on a other high popular science forum and asked about the same question, well the help was not good, felt like reply was "fuck you"
in a indirect way 
Quote: Originally posted by TheAustralianScientist  | | I don't think you need a result down to the millimole do you? I highly doubt you will get a margin of error above 1% unless you leave the NaOH out
without a lid for an hour and I think a percentage within 1% was all you needed am I correct? |
Hey im not working as chemist in pharmaceutical companies, im doing this for fun, and to learn, like a hobby. 1%-2% im MORE than happy. When i was
playing with the drain clear, i quess it was 80%, because the description said 60%-100% sulfuric acid, well my mixture dident end good as i want. So
the only thing i wanted is to know what % was in my drain cleaner bottle 
Quote: Originally posted by jsc  | I hope I caught you before you tried this.
Boiling sulfuric acid is a bad idea. What will happen is that a large amount of it will be vaporized. If you do it indoors, those vapors will then
condense on EVERY SURFACE IN THE HOUSE. For example, if you have wallpaper, this will result in large black splotches on the walls, giving it sort of
the "cow" look. It will also rust any and all metal in the house. Also, acids have a tendency to eat through clothes, so you wash your clothes and
there will be holes in them. Also, if you are in the house while those vapors are there you will be breathing those vapors. Get the picture?
|
I may be a newbie in chemist, but i was not born yesterday  , have seen 100 of
videos on youtube what sulfuric acid can do, so you think i would do it inside? Where i eat and sleep, nono m8. Hmm thought i wrote that i had a
portable hotplate, earlier in this thread , have seen 100 of
videos on youtube what sulfuric acid can do, so you think i would do it inside? Where i eat and sleep, nono m8. Hmm thought i wrote that i had a
portable hotplate, earlier in this thread  But thanks for reminding me of safety! But thanks for reminding me of safety!
About the NaOH stuff, (which seems like a war discussion here :p ). if it absorbs water, im okay. As long as the error of the acid concentraion is
between 1-2% 
But anyway, waiting for my PH meter to arrive, and i will do some test. Will keep you guys updated  
And thanks for all the attention and commitment!
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
Hey!
Sorry about the massive amount of disagreement thats been going on! As long as someone (possibly me) learns something from it, it's worth it.
In my opinion, I don't think the error will be more than 2% easy if you use NaOH, but if anyone else disagrees feel free to correct.
What is the other forum you were on? Yes, there are some great people on here, I have gotten my fair share of help from a lot of people.
Just found a two part video from NurdRage that explains about pH meters really nicely. You may have already seen but if not it is a great video to
learn from. (Part 1 and Part 2)
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
j_sum1
Administrator
       
Posts: 6326
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by TheAustralianScientist  | Hey!
Sorry about the massive amount of disagreement thats been going on! As long as someone (possibly me) learns something from it, it's worth it.
In my opinion, I don't think the error will be more than 2% easy if you use NaOH, but if anyone else disagrees feel free to correct.
What is the other forum you were on? Yes, there are some great people on here, I have gotten my fair share of help from a lot of people.
Just found a two part video from NurdRage that explains about pH meters really nicely. You may have already seen but if not it is a great video to
learn from. (Part 1 and Part 2) |
Yes, the error is likely to be more than 2%. That shouldn't be enough to stop anyone attempting a titration to get some practice at the technique.
It should be enough to stop someone being confident in the result.
@AusChem, if you have titrated H2SO4 against NaOH in the past and both have absorbed water, then the errors introduced will cancel each other out
somewhat. Reporting 98% H2SO4 has dropped to 97.5% is consistent with this. But I wouldn't hold a lot of confidence in that conclusion.
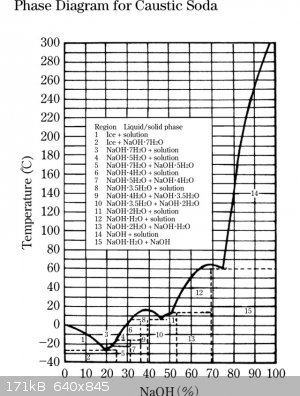
Phase diagrams... transport me to a happy place. You can gain a lot of information from a phase diagram if you know how to
use it. But more than a two minute explanation. What this particular one shows is that there are a lot of intermediate substances between 100%NaOH
and 100%water. In other words, NaOH absorbs water -- so much so that it eventually dissolves in the water it has absorbed. Nice find Bert. Care to
share your source?
@Trizocy. I would suggest that you will get much better results with an indicator than you will with a pH meter. Those things can be fickle. If you
don't have a buffer solution to store it in you can't expect the readings to be accurate. This is coming from someone who has ordered one recently
and is waiting impatiently for it to arrive in the mail.
Phenolphthalein is an excellent indicator for this purpose but if you don't have any then you can perform a very acceptable titration using cabbage
juice or squeezing the juice out of some coloured flowers. All you need to do is run some tests to determine what colours you are watching for in
acidic and alkaline environments.
Boiling down H2SO4 -- something I have done a bit of recently. Yes, do it outside. Yes expect anything steel in the vicinity to rust like mad. Yes
protect yourself against spills, splats and accidental elbow manoeuvres. A couple of months ago I did a beautiful job of cleaning my concrete shed
floor with a 1L beaker of boiling H2SO4. (I was a bit peeved at losing my favourite beaker too.) It pays to be careful. Yes, avoid breathing in any
fumes. Pull it off the heat when dense white fumes appear. All this is assuming it needs concentrating. Trizocy, it seems like your acid is strong
enough for most purposes without further concentrating.
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
Quote: Originally posted by j_sum1  |
Yes, the error is likely to be more than 2%. That shouldn't be enough to stop anyone attempting a titration to get some practice at the technique.
It should be enough to stop someone being confident in the result.
@AusChem, if you have titrated H2SO4 against NaOH in the past and both have absorbed water, then the errors introduced will cancel each other out
somewhat. Reporting 98% H2SO4 has dropped to 97.5% is consistent with this. But I wouldn't hold a lot of confidence in that conclusion.
Phase diagrams... transport me to a happy place. You can gain a lot of information from a phase diagram if you know how to
use it. But more than a two minute explanation. What this particular one shows is that there are a lot of intermediate substances between 100%NaOH
and 100%water. In other words, NaOH absorbs water -- so much so that it eventually dissolves in the water it has absorbed. Nice find Bert. Care to
share your source?
@Trizocy. I would suggest that you will get much better results with an indicator than you will with a pH meter. Those things can be fickle. If you
don't have a buffer solution to store it in you can't expect the readings to be accurate. This is coming from someone who has ordered one recently
and is waiting impatiently for it to arrive in the mail.
Phenolphthalein is an excellent indicator for this purpose but if you don't have any then you can perform a very acceptable titration using cabbage
juice or squeezing the juice out of some coloured flowers. All you need to do is run some tests to determine what colours you are watching for in
acidic and alkaline environments.
|
Hi j_sum. Thanks for your input. What base would you suggest for titrating H2SO4? Makes sense about them both cancelling each other out. Thankyou
for clarifying the phase diagram. I will look into them further.
Trizocy, I agree with j_sum, that if you don't have a buffer solution to store, you would be much better off with an indicator. Phenolphthalein is
pretty easy to find online so you should be able to find some if you look. Phenolphthalein is great because it has such a distinct colour change so
you can't miss the equivalence point.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by TheAustralianScientist  | Fine for a rough titration.
I also don't think that NaOH stored in an airtight container will be 10% water. If that was the case it would be a slush.
My mistake with the Suphuric Acid. You are right there. But if it's kept in an airtight bottle it will be fine. I have had 98% Sulphuric Acid for 6
months now and my last titration showed 97.5% so nothing serious has happened.
|
Where I worked at we had a large tank of 50% NaOH, it was a heated tank because the 50% NaOH would turn to a solid at cool temps.
You can clearly see this on the phase diagram Bert provided.
[Edited on 18-2-2015 by morganbw]
|
|
|
Trizocy
Harmless

Posts: 34
Registered: 16-2-2015
Member Is Offline
Mood: No Mood
|
|
chemicalforums.com
Quote: Originally posted by j_sum1  | | @Trizocy. I would suggest that you will get much better results with an indicator than you will with a pH meter. Those things can be fickle. If you
don't have a buffer solution to store it in you can't expect the readings to be accurate. This is coming from someone who has ordered one recently and
is waiting impatiently for it to arrive in the mail. |
http://www.aliexpress.com/item/Free-shipping-New-7-00-9-18-1... + distilled water?
Would this work out?
And do i need ph buffer for every ph or its okay to get 3ph 8ph and 12 ph?
Thanks
|
|
|
j_sum1
Administrator
       
Posts: 6326
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Suggestions for titrating H2SO4.
Now I have to confess to never having had to perform a highly accurate base titration. You want a base with the following properties
strong base for a well-defined endpoint
high purity
something that does not absorb moisture (or anything else) from the atmosphere
I believe Ba(OH)2 fits the bill but I am unsure how commonly that is used. [edit] Ba(OH)2.8H2O Barium hydroxide octohydrate [/edit]
The alternative is to use an acid standard to analyse your NaOH. Oxalic acid would work here and I believe that this is a common approach.
This is where the schoolteachers step out of the arena and the real chemists step in. Those with actual experience.
[Edited on 18-2-2015 by j_sum1]
|
|
|
j_sum1
Administrator
       
Posts: 6326
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
@Tizocy
Distilled water is exactly the opposite of what you want. A minute amount of contaminant (for example the remains of the last thing you measured)
will greatly alter the pH of the water. The pH could swing widely.
pH meters have a glass electrode. The should remain wet. And I think that they should remain at a constant pH when stored for any period of time. I
am not precisely sure what that pH is or whether it varies from model to model. The one at my work is sitting in a solution of pH 4.5 which came when
it was purchased.
A buffer solution is a solution of a weak acid and its conjugate base (or vice versa) that maintains a stable pH even when considerable amounts of
acid or base contaminant are added. It works on the basis that the weak acid does not dissociate fully. If some of that acid is neutralised by a
base then the equilibrium with the conjugate restores the solution close to what it was originally.
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
| Quote: |
I believe Ba(OH)2 fits the bill but I am unsure how commonly that is used. |
That would work, but like you said, I don't think it is very commonly available and its also fairly expensive IIRC.
| Quote: | | And do i need ph buffer for every ph or its okay to get 3ph 8ph and 12 ph? |
Just a single pH buffer will suffice (although you may need a certain one for your particular meter). Generally you buy it in liquid form, not sure
how those powders work.
(As a side note, generally you don't write "3ph" you write "pH 3".)
Personally I think a pH meter is more trouble than it's worth when you can use something like Phenolphthalein. Do you have access to that Trizocy?
| Quote: | The alternative is to use an acid standard to analyse your NaOH. Oxalic acid would work here and I believe that this is a common approach.
This is where the schoolteachers step out of the arena and the real chemists step in. Those with actual experience. |
I would be interested in learning more if a more experienced person happens to read this. I will do some reading myself as well.
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Trizocy
Harmless

Posts: 34
Registered: 16-2-2015
Member Is Offline
Mood: No Mood
|
|
Goal is not to get 99% accurate
NaOH pretty common in stores here, Ba(OH) have no idea where to get that :/
Here is the link of my pH meter  what u think? what u think?
http://www.aliexpress.com/item/3-in-1-Bench-Type-digital-PH-...
About the Phenolphthalein, im not sure :S
|
|
|
j_sum1
Administrator
       
Posts: 6326
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
No. But you might want better than 85% accurate.
Ba(OH)2 I don't think is that difficult to get. It is probably not the only (or even best) option. It is just the one that came to mind.
The pH meter looks a beast. Look after it. Find out what buffer solution it needs and get it. The temptation will be to think that the box on the
bench is the technical part. It isn't. The probe is what you need to look after.
Seriously, if you don't want to be bothered with buying indicators, have a play with some coloured flowers or vegetables. They are some of the best
indicators you can get. After all, litmus is merely extract from a lichen.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Still appreciating your post, either way  . .
Quote: Originally posted by TheAustralianScientist  |
Sorry if I sound stupid, but I'm not quite sure what that diagram is supposed to mean.
I freely admit there is MOUNTAINS of stuff I still need to learn and would be more than pleased if someone is willing to teach. Meanwhile, I like to
pass on what knowledge I do know to others in this forum where I can be corrected if wrong. |
You don't sound stupid at all. I wish more people in the forum were as polite and on point as you two when disagreeing, as it makes everything easier.
If one of us thinks it, someone else probably would too. I'll take a crack at Bert's diagram, and see if he was coming it from another direction:
Notice that you look at the dependent, or x-axis, you can start from the far right with nearly 100% pure NaOH. Look at the y-axis, and go to your
suggested temperature of 180 degrees. Now find the intercept. Because NaOH is deliquescent, it is still capable of forming an 80% solution essentially
unaided at this temperature. So, in an ideal environment, unless your NaOH was below 80% solution, I don't think heating to this temperature would be
helpful for drying. In practice, it would eat through your glassware and likely take on CO2 from the atmosphere, which happens to an extent at room
temperature. This carbonate formation would obviously introduce error into your titration.
That's how I chose to view it. As for hygroscopy on wikipedia... funny! I always used "hygroscopicity" as the noun form. Now I'm going to be thinking
about that all day!
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Oh Bert, what would I do without you...? I wonder how long I've been misspelling that word too...
Sorry to continue this sodium hydroxide hydrate discussion, but I might as well finish.
First, just think about it. Sodium hydroxide dissolves exothermically in water, why? I talked a little about this here. Quote: Originally posted by Molecular Manipulations  |
Water molecules are attracted to solid sodium hydroxide. Why? Water molecules are polar and sodium hydroxide is made of ions. These two are attracted
through the ion-dipole interaction, which is a Van der Waals force.
The positive part of the water molecule (the hydrogens) attract the negative hydroxide ion while the negative oxygen part attracts the positive sodium
ion.
But why does sodium hydroxide dissolve exothermically while say ammonium nitrate dissolves endothermically? Because all ionic crystals have lattice
structures. When a solid lattice forms, energy is released. When a substance is dissolved, the lattice is pulled apart and destroyed - this requires
energy. This is where Van der Waals comes in, there are three Van der Waals forces at play here. The "solvent-solute attraction", the
"solvent-solvent attraction" and the "solute-solute attraction".
The relative strengths of each force are critical in determining whether it will dissolve endothermically, exothermically, or not at all.
When the solute-solvent attraction is greater than the solute-solute attraction the substance will dissolve exothermically.
So in a sense you're right about the breaking of bonds (the crystal lattice). But it gets way more complicated than that, I've tried to simplify it as
much as possible.
|
That out of the way, why do you think sodium hydroxide is hygroscopic? For the same reason of course! The solvent-solute attraction
is very strong, when a water molecule in the air collides with it, rather than bouncing off like it would with most substances, Van der Waals forces
cause it to combine - permanently (unless it's strongly heated). Sodium hydroxide wants to dissociate, but without water, it's solute-solute
attraction (the crystal lattice) keeps it in place. So when I posted that it incorporates the water into it's structure, I had actually never seen
that before, it just made sense, it has to do that.
Check this out
Quote:
| Quote: |
Metal cations (being positively charged) attract the lone pairs on water oxygens and form coordinate covalent bonds with water. For example, many
divalent cations M2+ can form ions like [M(H2O)6]2+. One or more of the waters can be replaced with anions in the hydrate.
|
The water is bonded to the ions with covalent bonds, this releases a lot of energy.
That same link also answers Amos's question about activated alumina and maybe even dry dirt!
| Quote: |
Water molecules can diffuse into the material and become trapped there, especially if the material contains voids that can hydrogen-bond the water in
place.
Some porous materials can absorb large amounts of water by a phenomena called capillary condensation. For example, zeolites (complex sodium aluminum
silicates) are honeycombed with cavities lined with oxygen atoms. Water in air hydrogen bonds to the outer surfaces of the material. As more water
condenses, it tries to spread out and wet as much of the surface as possible to minimize its energy through hydrogen-bonding. That carries the water
deeper into the material, making more room for water to adsorb to the outer surface. (They soak up water like a sponge!)
|
Does this answer your question? I know I couldn't find as good of "proof" that it is incorporated into the structure as I thought I could, but this
seems good enough.
[Edited on 18-2-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
If someone were doing titrations to determine reagent concentration, something like these burettes could be handy- Assuming one had figured out how to
make or acquired a standard solution to dispense from such.
https://www.sciencemadness.org/whisper/viewthread.php?tid=61...

Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
What about density measurement? Isn't it precise enough to tell you how concentrated is your acid? Titration has it's problems since you need
titration standards (in ampules) to prepare solution of exactly known normality. once I also suffered with this same problem, I even found out an
article about usage of borax for titration (when recrystallized properly borax has very well defined "composition"), but never went to test it, maybe
anyone else did this?
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
NaOH is easily available in stores like Lowes as drain cleaner. Be careful though... it's better to get reagents from amateur chemistry suppliers such
as Elemental Scientific, because buying lots of chemicals from hardware stores is associated with cookery.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by Cou  |
NaOH is easily available in stores like Lowes as drain cleaner. Be careful though... it's better to get reagents from amateur chemistry suppliers such
as Elemental Scientific, because buying lots of chemicals from hardware stores is associated with cookery. |
If you're really worried, use cash. I've had no issue buying sulfuric acid, sodium hydroxide, toluene, MEK, and acetone all at the same time from
Ace.
|
|
|
Trizocy
Harmless

Posts: 34
Registered: 16-2-2015
Member Is Offline
Mood: No Mood
|
|
Hey, like i said, im not looking for 99,999999% acid, all i want to know is what concenration acid im playing with :/
Buying pure grade sulfuric acid here in my country is hard, you need to document/school/company. But the drain cleaner contains 60-100% sulfuric acid
so easy to buy 
Right now, im waiting for my pH meter : http://www.aliexpress.com/item/3-in-1-Bench-Type-digital-PH-...
And im looking for a burette, also buffer soultion. And NaOH which they sell everywhere. This will be okay?
Its okay to have 1-4% errors.
Damn when TheAustralianScientist told me about the NaOH, i thought i would be fun and easy, until the accurate guys came and says everything is
wrong,:" you will have to much error" 
Well thanks guys  ! !
|
|
|
j_sum1
Administrator
       
Posts: 6326
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
If you think I am discouraging you from having a go then you have misunderstood me. But you do need to realise ahead of time that the result you get
is likely to be more than a few percent off.
You might get better success in terms of accuracy if you use sodium carbonate: washing soda. It doesn't absorb moisture the same way. It does have
the disadvantage of being a weaker base and so you have to be a bit careful of the endpoint -- whether you use indicator or pH meter. And you get the
added irritation of CO2 bubbles in your titration.
If it was me doing it with the equipment you have then I would do both titrations.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Density measurements compared with charts of acid density vs. concentration is an OK way to determine acid %- IF you have an accurate graduated
cylinder and an accurate scale, plus are careful to use the cylinder near the calibration temperature. I have made the mistake of using standard
temperature calibrated graduated cylinders outdoors in below freezing weather (because I chose to boil down my sulfuric acid outdoors!)
I'd settle for within 3% accuracy in acid concentration measurements in most reaction processes. Besides analytical work, where do you need control
tighter than that?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Volanschemia
Hazard to Others
  
Posts: 340
Registered: 16-1-2015
Location: Victoria, Australia
Member Is Offline
Mood: Pretty much all of them!
|
|
| Quote: |
NaOH pretty common in stores here, Ba(OH) have no idea where to get that :/ |
I'm not really sure either. The only place i've seen Barium Hydroxide is on Ebay. Here is a link to a listing from a seller I have bought from but it is a bit expensive.
| Quote: | Here is the link of my pH meter  what u think? what u think? |
Wow! That is a nice meter. Make sure you look after it and it will serve you well.
Chemosynthesis, thankyou for your clarification on the phase diagram. Between yours and j_sum's explanation, I now understand.
| Quote: |
That's how I chose to view it. As for hygroscopy on wikipedia... funny! I always used "hygroscopicity" as the noun form. Now I'm going to be thinking
about that all day! |
 I like 'Hygroscopicity', it has a nice ring to it. It's very confusing with
the whole 'd' or 'g' thing! I like 'Hygroscopicity', it has a nice ring to it. It's very confusing with
the whole 'd' or 'g' thing!
Molecular Manipulations, thankyou so much for your effort in procuring that information. It seems we have both learned something
from this. So, it's not your standard hydration, but I admit that it is still a hydration and I was wrong.
| Quote: | | NaOH is easily available in stores like Lowes as drain cleaner. Be careful though... it's better to get reagents from amateur chemistry suppliers such
as Elemental Scientific, because buying lots of chemicals from hardware stores is associated with cookery. |
This may not hold true for Norway, but I have NEVER gotten a funny look from buying caustic soda (NaOH) from the supermarket.
| Quote: | | Damn when TheAustralianScientist told me about the NaOH, i thought i would be fun and easy, until the accurate guys came and says everything is
wrong,:" you will have to much error" |
Sorry for misleading you Trizocy. It seems that I still have a bit to learn myself...
| Quote: | | You might get better success in terms of accuracy if you use sodium carbonate: washing soda. |
Or Sodium Hydrogen Carbonate (Bi-carb Soda) would work as well. Just be aware though that both Na(CO3)2 and NaHCO3 will take more solution (due to it
being a weaker base) and I think (again feel free to correct anyone) that small amounts of Carbonic Acid will remain in solution and may skew results.
Measuring density may be your best option but I have little to no knowledge on the subject, so you would have to get your info from someone else.
Sorry about so many quotes, there were so many things I wanted to address.
[Edited on 19-2-2015 by TheAustralianScientist]
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and
vapor, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the
Persian king" - Johann Joachim Becher, 1635 to 1682.
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
For what it's worth, I use density measurements almost exclusively when trying to determine the concentration of acids and other substances. Sulfuric
acid is ideal for a density measurement, because the density of pure sulfuric acid is about 1.8.
A 100ml erlenmeyer flask and a decent scale will tell you the concentration of your acid to within a few %, and you don't have to mess around with
titrations and other such shenanigans.
|
|
|
| Pages:
1
2
3
4 |