| Pages:
1
2
3
4
5
..
22 |
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by cpman  | Does guano from the largest urban bat colony in the world count as OTC?
I'm only 4 miles from it and the soil under it is surely enriched with nitrates... |
yeah must be OK as it is OTC Out The Cave 
Dont ask me, I only know enough to be dangerous
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Having given our ghostly friend a while to play with chicken shit, I will now point out that their metabolism is different; they don't produce urea
and he may be barking up the wrong tree.
It's possible that the uric acid will be converted to nitrate by some bacterium or bacteria.
If it stinks of ammonia you should be OK
(that's not a phrase you get to use very often.
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by unionised  | Having given our ghostly friend a while to play with chicken shit, I will now point out that their metabolism is different; they don't produce urea
and he may be barking up the wrong tree.
It's possible that the uric acid will be converted to nitrate by some bacterium or bacteria.
If it stinks of ammonia you should be OK
(that's not a phrase you get to use very often. |
You have just given away three facts about yourself!
1) you have never had an allotment! allotment holder bug chicken keepers non stop for chicken shit. A
2) you have never ever ever cleaned out a medium to large chicken house!! if you dont do it often or use the deep litter method your eyes STREAM with
the ammonia
3) You dont know the white bit in bird poo is urea
 Chicken shit is world class fertilizer because of its extremely high nitrogen
content. Chicken shit is world class fertilizer because of its extremely high nitrogen
content.
Go visit a chicken farm man you can the ammonia for miles!!
Dont ask me, I only know enough to be dangerous
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
He doesn't for good reason, it's uric acid.
|
|
|
Mesa
Hazard to Others
  
Posts: 264
Registered: 2-7-2013
Member Is Offline
Mood: No Mood
|
|
At the risk of inviting ridicule at the incredible waste of an expensive resource, my first thought was to use the high alkaloid content of some trees
growing a block away from my house(A. Obtusifolia) and attempt some inefficient path back to NO2- from there. Probably less hassle to just steal a
kilo of kava from my Dad next time I'm around there.
Is this route a complete pipe dream? Or is there any feasability(Not withstanding the economic factors)
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Yeah ok I will give you that, the point still stands that chicken shit gives off plenty ammonia.
Dont ask me, I only know enough to be dangerous
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
From one of my favorite old school websites, a brilliant and simple resource for home grown nitrate:
http://www.musketeer.ch/blackpowder/saltpeter.html
The rest of that site about the science of black powder and the handgonnes (not a spelling error) is mind blowing (excuse the pun).
[Edited on 2-12-2014 by deltaH]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Ok, I have had a book mysteriously appear! Well the postman delivered it but I have not the faintest clue where it came from, Now I need a rule
check........... The book is Nitrogen as an Ecological Factor, by J.A.Lee etal.
Havnt read it yet (only just got it), now slightly scared! I might open it to find out chicken shit is just crap, or it might confirm my thoughts????
Hmmmmm
Anyway Thank you to whoever sent it!! I love my books, and ecology and chemistry combined is perfect. I have a feeling leaving Devon was a bad idea,
we had a deep litter system there. Here in Scotland the litter is only 12 months old, reading a few select pages does not sound like I am gonna get
the nitrate I want from it.
SHIT SHIT SHIT..........Marker pen back out and more drawing on the window!
Thanks for the book! U2U me if your responsible! Actually flicking through its a great book
Dont ask me, I only know enough to be dangerous
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Perhaps the book is full of Nitrogen ?
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Little_Ghost_again  | Quote: Originally posted by unionised  | Having given our ghostly friend a while to play with chicken shit, I will now point out that their metabolism is different; they don't produce urea
and he may be barking up the wrong tree.
It's possible that the uric acid will be converted to nitrate by some bacterium or bacteria.
If it stinks of ammonia you should be OK
(that's not a phrase you get to use very often. |
You have just given away three facts about yourself!
1) you have never had an allotment! allotment holder bug chicken keepers non stop for chicken shit. A
2) you have never ever ever cleaned out a medium to large chicken house!! if you dont do it often or use the deep litter method your eyes STREAM with
the ammonia
3) You dont know the white bit in bird poo is urea
 Chicken shit is world class fertilizer because of its extremely high nitrogen
content. Chicken shit is world class fertilizer because of its extremely high nitrogen
content.
Go visit a chicken farm man you can the ammonia for miles!! |
I never had an allotment- but I have a garden. I buy cheap fertiliser based on urea. I'm not prepared to make a big song and dance about using
"organic" fertiliser.
I grew up in a house next to a field full of chickens- but they were free range. I have been in a shed with 2000 young chicks- I don't remember it
smelling that bad (it was 40 years ago so I might not recall) Perhaps they cleaned it out more often
and, as has been pointed out, when I say that bird metabolism is different, you might want to look at my reputation here and check your "facts" before
telling me I'm wrong.
The last time I had occasion to check it was in the related issue of iguana droppings- it's a long story.
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by unionised  | Quote: Originally posted by Little_Ghost_again  | Quote: Originally posted by unionised  | Having given our ghostly friend a while to play with chicken shit, I will now point out that their metabolism is different; they don't produce urea
and he may be barking up the wrong tree.
It's possible that the uric acid will be converted to nitrate by some bacterium or bacteria.
If it stinks of ammonia you should be OK
(that's not a phrase you get to use very often. |
You have just given away three facts about yourself!
1) you have never had an allotment! allotment holder bug chicken keepers non stop for chicken shit. A
2) you have never ever ever cleaned out a medium to large chicken house!! if you dont do it often or use the deep litter method your eyes STREAM with
the ammonia
3) You dont know the white bit in bird poo is urea
 Chicken shit is world class fertilizer because of its extremely high nitrogen
content. Chicken shit is world class fertilizer because of its extremely high nitrogen
content.
Go visit a chicken farm man you can the ammonia for miles!! |
I never had an allotment- but I have a garden. I buy cheap fertiliser based on urea. I'm not prepared to make a big song and dance about using
"organic" fertiliser.
I grew up in a house next to a field full of chickens- but they were free range. I have been in a shed with 2000 young chicks- I don't remember it
smelling that bad (it was 40 years ago so I might not recall) Perhaps they cleaned it out more often
and, as has been pointed out, when I say that bird metabolism is different, you might want to look at my reputation here and check your "facts" before
telling me I'm wrong.
The last time I had occasion to check it was in the related issue of iguana droppings- it's a long story. |
Well you took that the wrong way or got out of bed the wrong side!! Chicken sheds not cleaned properly or not using deep litter stink of ammonia, I
dont know or care what causes it, but its there. Using chicken shit on a garden has nothing to do with organics, its simply one the best ferts you can
use.
I am really sorry to hear your not often wrong, and I hope this time hasnt traumatized you too much  . .
I respect you BUT I am soooooo sure on chicken shit lets go dig and see who is correct! If I am wrong then I will eat a cup of it on video.
ours free range as well, but we get plenty poo over night under the roosts, we swapped to deep litter ages ago and you get a fantastic compost, but
the garden people like it raw and like to rot it down with hay, we use saw dust.
Thats given me an idea with sand and a bucket!
As a side note what would the best way to analyze chicken shit with a GC be? I could then quantize the amount per g of each component, then compare
with some thats started to compost.
Dont ask me, I only know enough to be dangerous
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
It is uric acid yes, not urea, and it does indeed decompose to ammonia.
http://www.academia.edu/1799968/AMMONIA_EMISSION_FROM_POULTR...
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I read the regular updates in this thread with great enjoyment. While the concept of harvesting nitrates from bird crap is a fascinating one, it
doesn't personally appeal to me. I suppose this is due to the need for handling, well, crap. I have a family of cats living with me right now (and I
can't seem to get rid of them). Once I scoop the remains of whatever I fed them the night before from their litter box, I never want to see that crap
again.
I'm more of an electronics guy than anything else. I live my daily life in a lab surrounded by cabinets full of electronic components and test
equipment. Personally, I'm going to go the Birkeland-Eyde route. Maybe it won't fit the rules of the competition, but then again, I wouldn't accept
money even if I won anyway. If someone else thinks they can do this better than me, then feel free to jump in there and do it. I'm not claiming
pre-eminence on this particular idea. I plan on converting 120VAC to DC, then converting to a high frequency sine wave. The high frequency is to
keep the arc plasma from extinguishing between half cycles. The output voltage would be about 60VAC open circuit, but the voltage should rise across
the arc when it gets blown out by the magnetic field.
All of the parts would be common, available from multiple sources, and fairly inexpensive. The idea isn't, "Now buy an expensive transformer".
Instead, the idea is, "Buy these ordinary components from one of several suppliers, and make your own transformer."
In the interest of designing something that other people can duplicate, I'll deliberately keep the design simple. I can design with parts that are
available from suppliers in both the US and Europe. If anyone else has a favorite supplier in a different country, I can try and make sure that the
components can be sourced from them as well.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I'll share my hypothetical idea for achieveing this task for others to use if they so desire.
The combustion of fuel results in the production of nitrogen oxides. Of the many mechanistic sources by which these may be produced, so-called 'fuel
derived NOx' can produce the most if combustion is carried out with excellent airation, AFAIK. Fuel NOx is NOx that is produced from nitrogen atoms in
the fuel molecule itself, for example, from burning amines.
So hypothetically, burning a very nitrogen rich fuel should produce lots of NOx in the flue gas which can be used to make nitric acid by subsequent
absorbtion and oxidation.
Beware though that burning nitrogenic fuels can also produce cyanide, but I think this is more an issue when the combustion is carried out with
insufficient air.
Theoretically, one could use fuel tablets as a nitrogenic fuel (from the hexamine component), however, these aren't exactly cheap. Soybeans, what with
their ~36% protein content, IS a cheap bulk nitrogenic 'fuel' 
I know this is very hypothetical, but I don't think it's too crazy, or is it?
[Edited on 4-12-2014 by deltaH]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
It might be worth a try. It is probably a bit more practical than some of the ideas that I have been throwing around. I wouldn't expect yield to be
that high though. And to get it to work well you would probably want a good oxygen supply -- richer than air would be beneficial I would imagine. It
could take a while to make the required 100mL. Of course bubbling through H2O2 will lift the yield a bit.
Purity might be an issue too. What other combustion products might come off the soya beans that could dissolve in solution?
I'm going to mull these things over.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Well I was thinking that for a large apparatus, one could have a suitable steel tank as a combustion chamber in which a large radial and horizontal
metal fan sits at the bottom and beans are fed in through the top, somehow.
A blower blows air in tangentially at the base, close to the radial fan. The beans are constantly bashed and tossed around by the spinning fan, this
ensures that they burn well with excess air. It would also break off any ash forming on the bean, further improving aeration. The exhaust gas outlet
is from the centre top, the spinning solids are flung outwards off course, so that should keep the exhaust free of big bits, but probably not the fine
ash. Ideally the exhaust should run into a cyclone to spin out the ash and then the gases fed to an absorber, the simplest of which could be made with
H2O2, though use air as the oxidant would be the cheapest, but hardest absorber to get to work.
As for other gases, N2O is possible, but that wouldn't absorb. SO2 probably as well? I don't know how much sulfur is in soy beans, but I guessing
quite a bit... but that shouldn't be a problem. The crude nitric acid formed probably would need distillation to clean it up anyway.
This kind of rig I'm proposing is for litre scales, not 100's ml, but perhaps a proof of concept should be explored with something very small first.
I don't know how easily fuel tablets blow out with wind, are they very resistant? If so, a simple test could be a burning fuel table in a metal tube
with a hair drier in the one end and the exhaust lead via a stopper and tube to bubble through H2O2 solution?
[Edited on 4-12-2014 by deltaH]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I have worked with fuel tablets before. (As in, I have cooked with them.) They burn quite steadily and don't blow out too easily. They aren't pure
hexamine though. They usually contain quite a bit of trioxane as well. This helps them burn steadily but does nothing for your NOx output.
Their appeal as a cooking fuel (or one of their appeals anyway) is the fact that combustion is very controllable by adjusting the amount on the pile.
A large pile of lumps will give a high temperature. Scatter your pieces a bit and the temperature goes down quickly. It burns cleanly with no smoke
except when the amount combusting is small and the temp low. I guess this means predictable or at least constant combustion products. I have never
tried blowing a hair drier onto them. (Couldn't find an electrical outlet in my tent.)
With soy beans I doubt there is much need to tumble roast. I would have thought a barbecue kind of arrangement would work, possibly with a vacuum
cleaner or similar blowing air through the pile. It should be possible to get a nice glowing pile of beans and direct the exhaust gases through an
absorber. A bucket of water might suffice.
I get the feeling that I am missing something here but it seems feasible.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
The trioxane isn't so much an issue as the idea to use fuel tablets is for proof of concept only, i.e. making some small amount of dilute nitric acid.
Making any significant amounts will require scaling up. My soy bean idea is just the cheapest OTC nitrogen-rich fuel I could think off.
I think what is going to make or break this is the selectivity for making nitric oxide. That is to say, what fraction of nitrogen atoms in the fuel is
converted to nitric oxide? It's probably poor, but if it's easy to carry out and the feedstock is cheap enough, who cares for an amateur setup?
Anyhow, I don't think this is too far removed from what is practiced industrially, i.e. industry burns THE most nitrogenic fuel... ammonia. However,
typically a catalyst like platinum is used in that case and I don't think that is practical in the amateur context.
It might not be necessary to fully agitate/fluidise the beans in the combustion chamber, but I suspect this might give the best results. As you said,
blowing lots of air through a packed bed of burning beans would probably also work, maybe close to just as well 
Another key factor may be how the speciation of the nitrogen in the fuel affects the selectivity to it forming NO. Perhaps the amides in proteins
don't work well at all compared to amines.
[Edited on 4-12-2014 by deltaH]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by deltaH  |
Anyhow, I don't think this is too far removed from what is practiced industrially, i.e. industry burns THE most nitrogenic fuel... ammonia. However,
typically a catalyst like platinum is used in that case and I don't think that is practical in the amateur context.
|
This was pretty much my idea. Decompose some urea. Produce some oxygen and then combust. But the reaction conditions are not straightforward.
High(ish) temperature, pressure at several atmospheres, platinum catalyst, low pressure needed for the second stage of the reaction and separation of
the undesirable gases to feed back through the system. It wasn't going to be simple.
I found some OTC platinum but at least in my part of the world it stopped being OTC about 15 or 20 years ago. I am surprised they are still available
anywhere. Contact lens technology has moved on.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Anyhow, bottom line, I am hoping one can burn protein in excess air to make some amount of NOx... hopefully in usable amounts. The calorific content
of soy beans is high, so the flame temperature under forced air will surely be very high, hopefully this will help with making the NOx in the absence
of catalyst.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
At 15% nitrogen, this is a possibility too.
http://www.texascollaborative.org/hildasustaita/module%20fil...
Might not burn as easily though.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Or old wool? Expensive though, but not if it's a moth-eaten jersey 
[Edited on 4-12-2014 by deltaH]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Like I said, tricky to burn.
I think vegetable protein as you have suggested is probably a more sensible option.
Anyway. Good food for thought.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Started digging the literature... interesting stuff.
From the book "Pollutants Generated by the Combustion of Solid Biomass Fuels" by Jenny M. Jones, et al, p54:
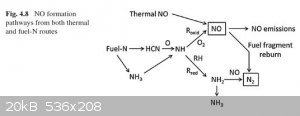
The book was only partially available for reading on google books. From page 55:
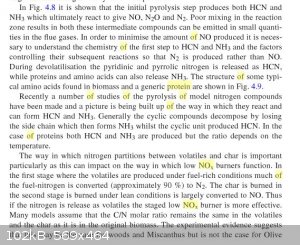
So it seems important that the combustion be well mixed to avoid producing small amounts of cyanide in the flue gas.
Anyhow, now at least we know burning protein produces NO... exactly how much is the next question.
[Edited on 4-12-2014 by deltaH]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Little_Ghost_again  | | As a side note what would the best way to analyze chicken shit with a GC be? I could then quantize the amount per g of each component, then compare
with some thats started to compost. |
GC as in Gas Chromatography? Ok, calm down already! Easiest way to determine ammoniacal nitrogen (i.e. ammonia + ammonium salts) would be dry
distillation of a know amount of chicken shit with an excess of solid NaOH. That will drive off all ammoniacal nitrogen as ammonia, quantitatively.
Capture the NH3 in water (quantitatively), dilute appropriately and determine the amount with acid/base titrometry (HCl titrant + methyl orange
indicator).
Constructing a neat little apparatus for the quantitative dry distillation of the NH3 is a doddle.
[Edited on 4-12-2014 by blogfast25]
|
|
|
| Pages:
1
2
3
4
5
..
22 |