| Pages:
1
2
3
4
5 |
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
being snowed in i collected some outside and melted it add some more melted it etc...
after collecting about a gallon of water i boilled it down to about 300 ml and pour 50ml in a small glass bottle that fit the dector...
started the acquisition and went to bed.
after 5 hours of collection this what i had.
<a href="http://www.scimad.org/users/neptunium/5_h_snow.jpg"><img src="http://www.scimad.org/users/neptunium/5_h_snow.jpg"
width="200"></a>
i thought that little hump was Cs137 at 661Kev from the Fukushima accident (hlf life 30 years) but no .
the software indidcated I131 and Bi214 .
i am not sure what it is actually but its definitely there.
the other peak left little doubt iT IS K40...
but i found it interesting because not too many element have a peak at 632Kev...
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: hosted
image(s) at scimad.org/scipics2/]
[Edited on 2.2.14 by bfesser]
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by neptunium  | | after collecting about a gallon of water i boilled it down to about 300 ml and pour 50ml in a small glass bottle that fit the dector...
|
Did you acquire a baseline on the empty bottle?
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
yes i did but it should have been 5 hours too to be accurate and i only did 1 hour...
<a href="http://www.scimad.org/users/neptunium/1_hour_blank.jpg"><img src="http://www.scimad.org/users/neptunium/1_hour_blank.jpg"
width="200"></a>
the K40 is barely visible because i was not standing there . in this basement (my lab) the main source of K40 is me !
i will get some lead shielding as soon as i get some money again!
whatever is in there is drowned in background radiations...
[Edited on 2-2-2014 by neptunium]
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: hosted
image(s) at scimad.org/scipics2/]
[Edited on 2.2.14 by bfesser]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
what hapen to the charts?? does my browser needs updates ? or is everything gone ?
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I can't see them.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i have a new detector much more performant with a better resolution,
i`d like to post graph as updates but i dont know what happened to the older ones....
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
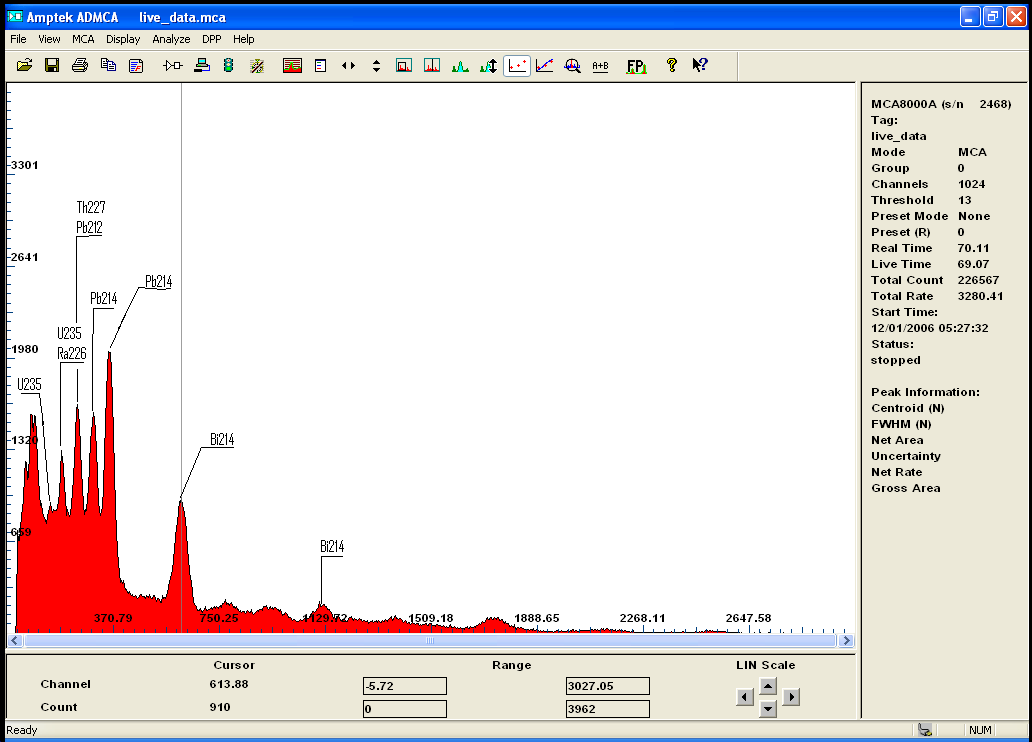
i recently acquired some modest quantities of the mineral called monazite . A source of rare earth and Thorium...
The gamma spectrum with my new improved detector clearly shows the isotopes from the decay chain of Th232
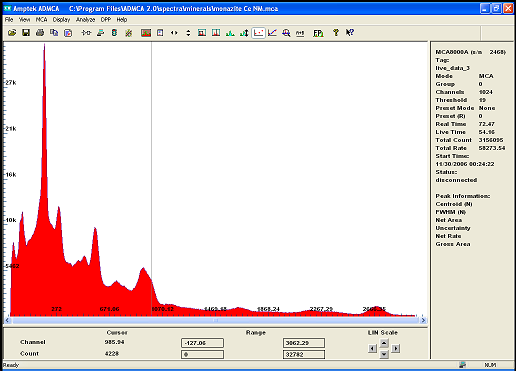
after calibration the main peak is label as U235 or Ra226 wich doesnt really matter because if one is present we can deduce it either came from the
other or the other must be there too .. also identified are isotopes of Pb, Bi, and Ac!
Also i have a small amoun of Euxenite ..
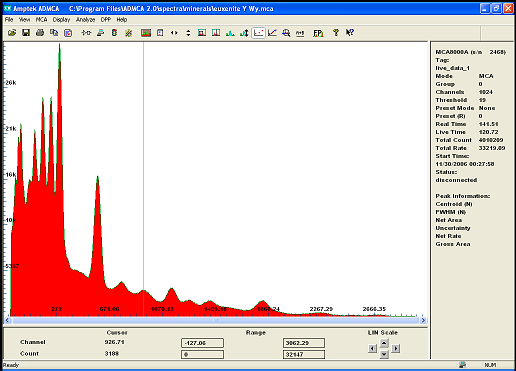
notice how the same peak appear on both spectrum indicating the presence of the same isotope..i beleive it to be Bismuth
i will redo the calibration with more care to be sure ...
but i find it quite interesting that U235 is clearly present in both rocks in much greater amont in Euxenite though
i have enough to seperate uranium ,Thorium and other element chemicaly but probably wont do it .
although it would be interesting to see how the spectrum changes...
[Edited on 20-8-2014 by neptunium]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
the previous spectrum of Co60 was somehow erased..
this is what my new detector resolution is able to accomplish!
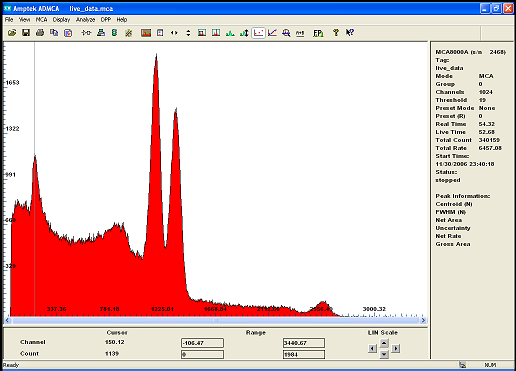
the two main peak at 1173.24Kev and 1332.5 kev are clearly seperated the small one over at 2.5Mev is just an addition peak.
The lower energy peak is the back scattering ( i need to built a shield)
and here is Barium 133 ...
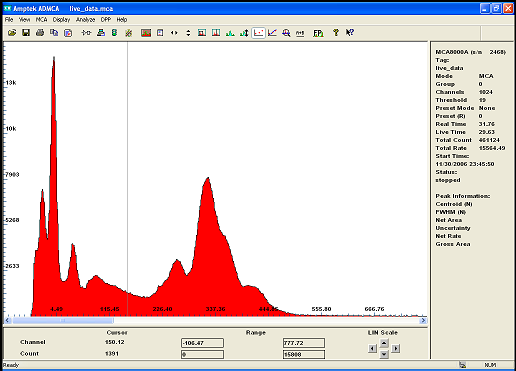
i get a much better resolution with the semiconductor detector but the surface area window being so small, it barely see higher energy gamma...it is
in fact an Xray sopectrometer.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
here is the identification of a sample of euxenite...
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
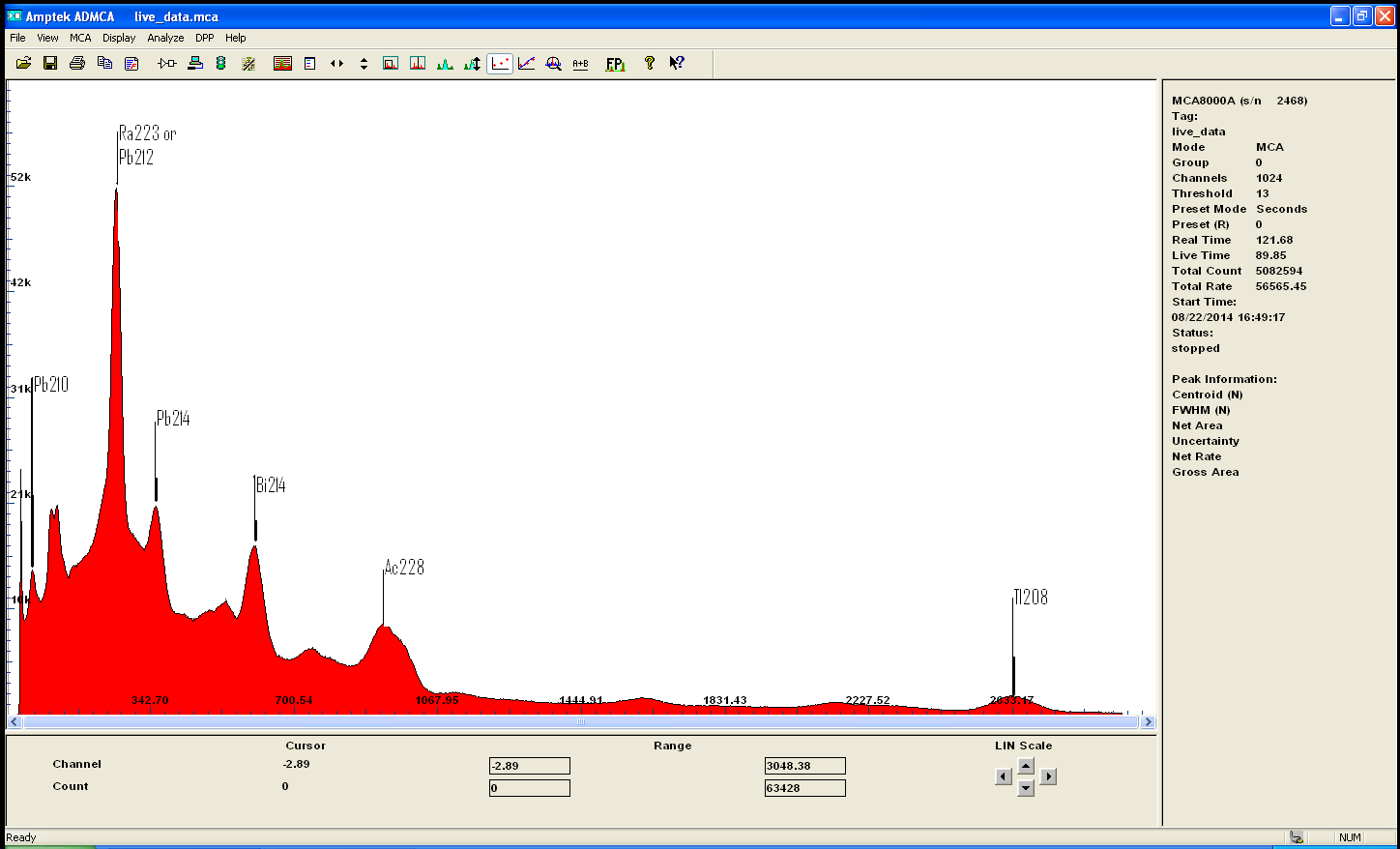
here is monazite
interestingly enough, Ra223 is a daughter of U235 with a half life of 11 days whereas Pb212 come from Th232 wich is more likely since this is
monazite...but with a half life of only 10 hours ... my calibration might need an update!
|
|
|
Polverone
Now celebrating 21 years of madness
|
Thread Moved
11-9-2014 at 19:17 |
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
You should post some of your spectra as a reference on the sciencemadness wiki! They would be very useful.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
great idea! thanks Volatile.!
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Sure!I'm thinking about getting the equip. for this within the next few years.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
from personal experience the most important piece is the detector. if you go with a cheap one you wont get the resolution regardless if you go semi
conductor or scintillator.
after that there is plenty of amplifier counter and software (free) to get a good clean signal processed.
good luck and have fun! i know it is for me!
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
I have order a steel shield inside of what i have melted some lead for the detector...
it cost me way more than i thought it would and left a bitter taste in my mouth.....untill

I am not finished with the lead melting process (over 200lbs is involve) the whole set up is looking a lot better than before .

and i had some interesting results with a substance i would never have suspected to contain any active material..
Zirconium dioxide
here is the gamma spectrum of about 2 lbs of ZrO2.
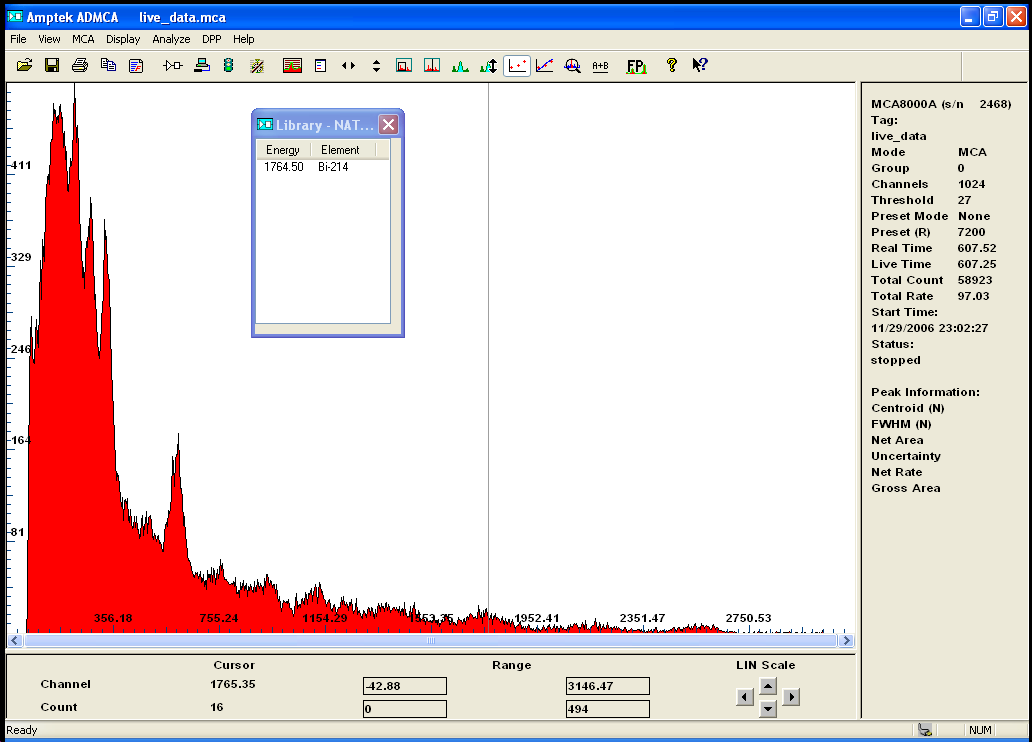
after careful calibration i was able to identify the natural element involve in the weak radio activity of this otherwise neutral substance...
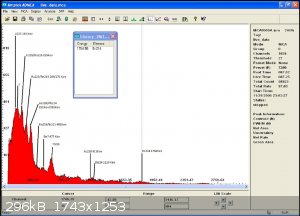
click on the picture for better viewing.
I am running an X-ray spectrum right now but the detector being way more sensitive and much smaller this will take a while .Each peak will be a lot
more defined but it only reads the spectrum untill about 330Kev ...so more results to come.
i beleive the chemical similarity of group 4 with the lanthanides (and actinides) allows some of those metals to be present in each other ore...
it would be interesting to see if they could be detected in Titanium and Hafnium salt as well...
we already know how Yttrium and some rare earth are often found with Thorium and vice versa..
maybe Zirconium is a big part of the impurities in Uranium processing..
[Edited on 8-8-2015 by neptunium]
|
|
|
annaandherdad
Hazard to Others
  
Posts: 387
Registered: 17-9-2011
Member Is Offline
Mood: No Mood
|
|
neptunium, this is very interesting! I did not know of the existence of this thread.
Where did you get your monazite?
I fear that your spectrometer is too costly for my budget, but I'd love to be able to play with such a thing.
Any other SF Bay chemists?
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
there is (was?)a guy on ebay selling it for cheap...
I acquire every pieces over the course of a year or 2 ... it was a long process but it paid off! some parts are cheaper than others thats true...
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
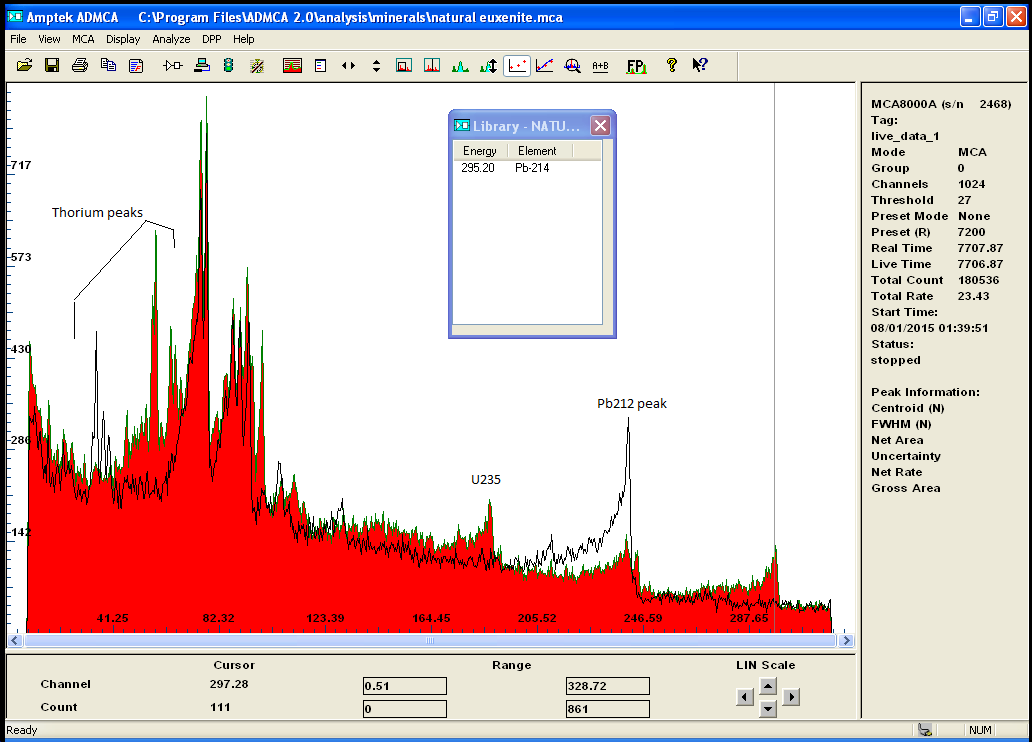
Monazite is a Thorium rich ore but still contain small amount of Uranium, when Euxenite is clearly a Uranium rich mineral.
the gamma spectrum clearly confirmed the presense of both Th and U and the decay chain of each isotopes and also quantify
those metals ..
here is the 2 spectrums for comparaison
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by neptunium  | there is (was?)a guy on ebay selling it for cheap...
I acquire every pieces over the course of a year or 2 ... it was a long process but it paid off! some parts are cheaper than others thats true...
|
I just bought 1 lb of monazite ore for $21 on eBay $15/lb plus $6 shipping), it was sourced to New Mexico.
If any SM members live in North Carolina there is a river that is a good source of monazite sand, much easier than gold panning. Unlike the ore
(perhaps), monazite sand is usually concentrated monazite. I am sure monazite sand would find willing buyers here.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
yeah!.........you should send me a sample!
|
|
|
Texium
Administrator
       
Posts: 4579
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by careysub  | Quote: Originally posted by neptunium  | there is (was?)a guy on ebay selling it for cheap...
I acquire every pieces over the course of a year or 2 ... it was a long process but it paid off! some parts are cheaper than others thats true...
|
I just bought 1 lb of monazite ore for $21 on eBay $15/lb plus $6 shipping), it was sourced to New Mexico.
If any SM members live in North Carolina there is a river that is a good source of monazite sand, much easier than gold panning. Unlike the ore
(perhaps), monazite sand is usually concentrated monazite. I am sure monazite sand would find willing buyers here. |
I've taken a look at that area of NC too. It looks very promising indeed. I know that there's a couple of members who live within a
few hours of there. I mentioned it to them once in the Skype group a while back, but didn't discuss it a whole lot. I'll bring it up again and see if
they're interested in it. I'd definitely like to get a sample too.
|
|
|
annaandherdad
Hazard to Others
  
Posts: 387
Registered: 17-9-2011
Member Is Offline
Mood: No Mood
|
|
Hi, careysub, I just bought 1lb of that monazite, too. It was a good deal.
A while back I spent a lot of time thinking about thorium, and I looked for monazite. There was none available, so now it's surprising to see it. I
did find some thorium nitrate reagent, and bought a small amount.
To tell you the truth, I hesitated to buy the monazite, since I'm not sure I'll use it for anything and the radon is a concern. Also, some day
someone will have to dispose of it. Actually it was the radon which caught my interest in the first place, since it has a dramatic effect in a cloud
chamber (this is the radon in the thorium decay chain, not the one in the uranium chain).
But something tells me it's going to get harder and harder to get things like this, so I decided to take it.
Any other SF Bay chemists?
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by annaandherdad  | Hi, careysub, I just bought 1lb of that monazite, too. It was a good deal.
A while back I spent a lot of time thinking about thorium, and I looked for monazite. There was none available, so now it's surprising to see it. I
did find some thorium nitrate reagent, and bought a small amount.
...
But something tells me it's going to get harder and harder to get things like this, so I decided to take it. |
You got that right. The seller tells me that the monazite deposit has been assayed with microprobe technology at 7-9.5%. This is by far the cheapest
source of thorium I have seen in the couple of years I have been looking.
Here are some papers on this monazite source:
https://nmgs.nmt.edu/publications/guidebooks/downloads/62/62...
http://geoinfo.nmt.edu/publications/periodicals/nmg/10/n2/nm...
http://deepblue.lib.umich.edu/bitstream/handle/2027.42/32413...
http://pubs.usgs.gov/sir/2010/5220/downloads/SIR10-5220.pdf
Keep your monazite in an airtight box (or outside, it is after all, a rock). The radon risk is nothing to sneeze, but you simply need to have proper
storage. The risk from thorium radon is much less that with uranium due to the very short half-life (55 seconds) but thorium ore often contains
uranium as well. The Petaca monazites apparently always have less than 1% U however.
[Edited on 10-8-2015 by careysub]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
here is for your consideration Radium 226 (old Radium dial...)
some daughter of Ra226 are visible (nevermind the cursor on Tl)
giving Radium`s half life this should be interesting ...
the scale at the bottom has been calibrated.
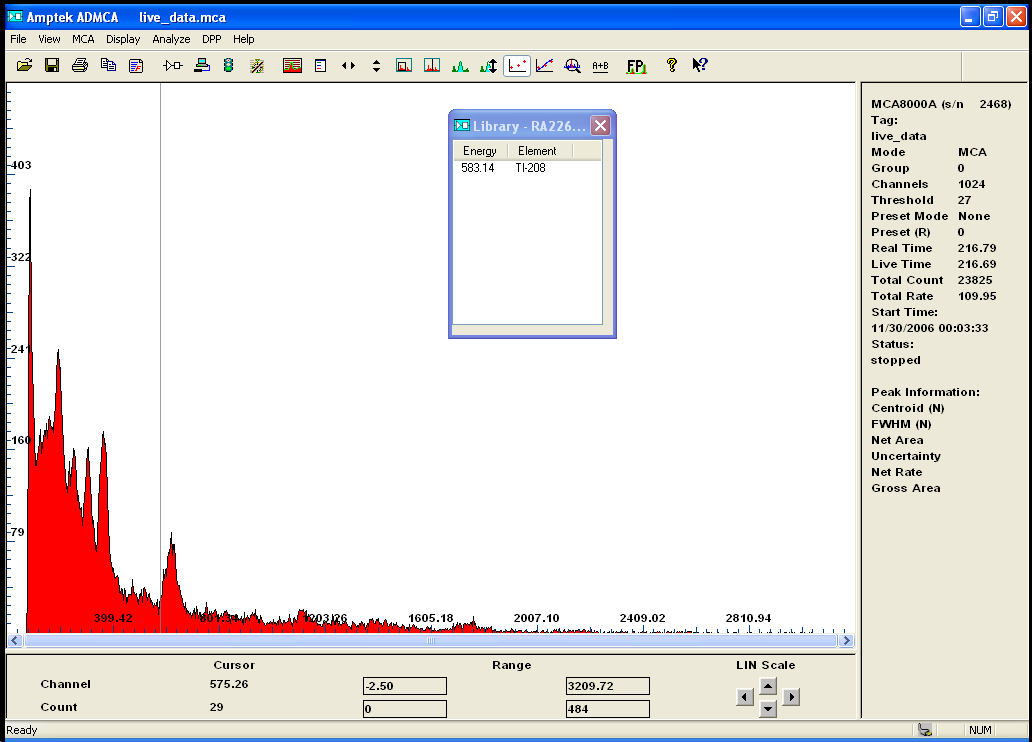
[Edited on 1-10-2015 by neptunium]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Very nice work! I live in Ohio, perhaps eventually a trip to NC would be fun.
|
|
|
| Pages:
1
2
3
4
5 |