| Pages:
1
2
3
4
5
..
18 |
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
page 2

|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
page 3
Page 4 is coming soon, one of my copies of it is not working so I have to find a working copy of it somewhere in my files.

|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
OTC azide?
I was reading Ullmann's yesterday, and it mentioned that barium azide is used "in fluorescent light tubes to improve the light yield."
This could be a viable source for azide depending upon how much barium azide is used in a fluorescent tube. Of course, removing the azide would be
quite dangerous as when the glass is broken barium azide would be spread into the air, as it is most likely used as a fine powder coating.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Copper (II) Azide
Darkflame mentioned copper azide above.
According to Brauer, when damp with H2O, copper II azide is fairly insensitive. However, when wet with ether (used for drying), or completely dry, it
is relatively sensitive, explosion temperature is at 215 deg C.
More importantly, it is six times (!!) more potent than lead azide as an initiator, 450 times more potent as mercury fulminate!
(M. Straumanis u. A. Cirulis, Z. Anorg. Allgem. Chem. 251, 315 (1943).)
I can translate the prep if desired. Although it is not radically different to the Pb one.
It doesn't need dextrine to avoid big crystals though. Just simple metathesis between copper nitrate and sodium azide, and additional washing
with HN3 ( ), and immediate washing with ethanol and then ether. ), and immediate washing with ethanol and then ether.
[Edited on 13-4-2005 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Oh dear...
I just realized I never posted info on copper azide for darkflame. I apologise. I apologise.
You might find these of interest as well Chemoleo.
Overall I like the idea of copper azide, especially because I dislike explosives that release finely divided poisonous metal into the air.
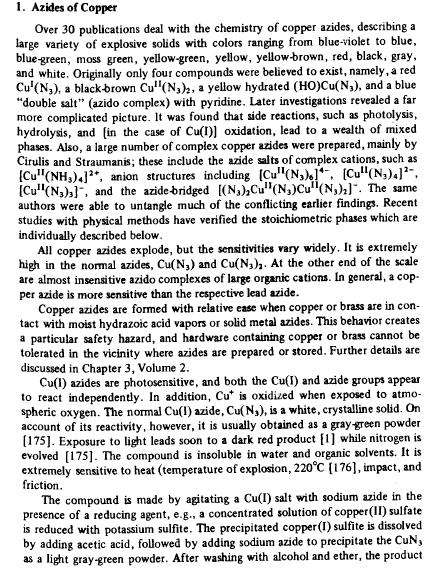
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
page 2

|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
page 3

|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
| Quote: | Originally posted by heksogen
I know that lead azide is made by mixing solutions of lead nitrate, sodium azide and dextrin and sodium azide is made by bubling nitrogen oxide throw
molten sodium amide. My question is :
Are there any other ways to get sodium or hydrogen azide??? |
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Preperation of Azides
Please install eMule 0.45b and search for "BRAUER" Handbook of preperative inorganic chemistry vol. 1-2. In this work of art, you will find
the synthesis of Azides, and many more inorganic explosive substances.
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
Pardon me, if I have asked before, however; Could dilute hydrazoic acid or ammonium azide not be formed by bubbling N2O through warm ammonia solution,
by the reaction
NH3 + N2O --> H2O + HN3
?
[Edited on 16-4-2005 by halogen]
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
You will grow a very long beard in the process, no insult intended. Please check out how molten Na (a violent reductor) reacts with NH3 to form NaNH2
(amide), with the evolution of H2 gas, and you will see why. N2O is relatively inert, and will find itself in an uphill battle with NH3 in respect to
plucking of a hydrogen atom.
Molten NaNH2 however, gives a different story.
[Edited on 17-4-2005 by Lambda]
[Edited on 17-4-2005 by Lambda]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
This one doesn't use sodamide or nitrites, so I put it here regardless of what it does use - NCl3. It is Ber. 32, 1399 (1899)
Diese Säure entsteht bei der Einwirkung des Chlorstickstoffs auf
Hydrazin. Seitdem Hentschel gezeigt hat, dass man eine Benzollösung
des Chlorstickstoffs gefahrlos herstellen und handhaben kann,
wird wahrscheinlich diese bisher gefährliche und gern vermiedene
Verbindung in den Kreis der gebräuchlichen Reagentien treten.
Ich nehme dreimal mehr Benzol, als Hentschel vorschreibt und bekomme
also eine 3.3-procentige Lösung von Chlorstickstoff. 30 ccm
dieser Lösung habe ich mit der kalten wässrigen Lösung von 1.5 g
Hydrazinsulfat im Scheidetrichter unter öfterem Schütteln zwei Stunden
zusammenwirken lassen. Hierauf habe ich die wässrige Lösung mit
Natronlauge genau neutralisirt, 10 ccm normaler Schwefelsäure zugesetzt
und ein Viertel der Flüssigkeit abdestillirt. Das saure Destillat
giebt mit Silbernitrat sofort einen weissen Niederschlag, der in Salpetersäure
sich vollständig löst. Bin Körnchen des trocknen Salzes
explodirt beim Erhitzen sehr heftig. Zweifellos ist es Stickstoffsilber,
AgN3. Die Ausbeute ist aber nur klein: ich habe in zwei Versuchen
5 pCt. und 6.5 pCt. der theoretischen Menge an Stickstoffwasserstoffsäure
erhalten. Im zweiten Versuch habe ich nach der
Reaction die Benzollösung mit 20 ccm Wasser gewaschen; vielleicht
ist es diesem Umstande zuzuschreiben, dass dabei die Ausbeute etwas
grösser ausfiel. In der Benzollösung bleibt noch viel unveränderter Chlorstickstoff.
Bessere Resultate lassen sich erzielen bei der Einwirkung von
Chlorstickstoff auf freies Hydrazin. Ich verfahre wie oben beschrieben,
nur mit dem Unterschiede, dass ich von Zeit zu Zeit 10-procentige
Natronlauge in kleinen Portionen (3—5 ccm) in den Scheidetrichter
gebe, bis die wässrige Lösung dauernd stark alkalisch reagirt. Im
Ganzen werden 30—35 ccm Lauge verbraucht. Die Operation dauert
unter öfterem Umschütteln 1 1/2 Stunden. Die wässrige Lösung
wird jetzt mit Schwefelsäure neutralisirt und nach Zusatz von 10 ccm
normaler Schwefelsäure ein Viertel der Flüssigkeit abdestillirt. Das
Destillat enthielt ein Mal keine Salzsäure, ein anderes Mal nur Spuren
davon. Acidimetrisch und dem Gewicht des Silbersalzes nach wurde
bestimmt, dass die Ausbeute an Stickstoffwasserstoffsäure 36 pCt. der
theoretischen erreichte, auf 1 g Chlorsticketoff gerechnet. Aus der
titrirten Säure bekam ich das Silbersalz und bestimmte darin das
Silber; gefunden wurden 71.13 — 71.03 pCt. Silber, während Silbernitrid
71.92 pCt. Silber enthalten soll.
Da bei der Einwirkung von Chlorstickstoff auf Hydrazin Gase
(Stickstoff?) und andere Nebenproducte nicht in auffallender Menge
entstehen, so glaube ich, dass die Ausbeuten an Stickstoffwasserstoffsäure
beim Arbeiten in grösserem Maassstabe höher ausfallen werden
und dass die beschriebene Darstellungsweise dieser höchst interessanten
Säure in manchen Fällen gebraucht werden kann. Bei der
Einwirkung von Kaliumnitrit (Angelo Angeli) und von Salpetersaure
(Sabanejeff) auf Hydrazinsalze entstehen nur kleine Mengen der
Stickstoffwasserstoffsäure, nur die Methode von Wislicenus giebt bessere Resultate.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I am interested in such a novel route, however my level of German courses is not high enough for me to understand the majority of that. Could you
post a translation?
EDIT: I used a online translator and got the gist of it, but a potentially serious number of nonsense phrases and untranslated words resulted.
[Edited on 16-4-2005 by rogue chemist]
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
Translation
I will translate this preperation, and get it up and running on monday.
|
|
|
redneck
Harmless

Posts: 12
Registered: 26-9-2004
Member Is Offline
Mood: No Mood
|
|
Hey S.C.WACK you live in the states, so where did get such an old german chemistry boock? Do you speak german?
Translation:
This acid develops during the effect of the nitrogen trichloride on hydrazine. Since Hentschel showed that one can manufacture and handle a benzene
solution of the nitrogen trichloride safely, this dangerous and gladly avoided compound will probably step into the circle of the common reagents. I
take three times more benzene, than Hentschel prescribes and get thus a 3.3-percentage solution of nitrogen trichloride. I let 30 ml of this solution
react with the cold aqueous solution of 1.5 g hydrazine sulfate in the separating funnel under frequent shaking for two hours. Then I exactly
neutralised the aqueous solution with a caustic soda solution, afterwards I added 10 ml of normal sulfuric acid and distilled over a quarter of the
liquid. The sour distillate immediately forms with silver nitrate a white precipitation, which solves completely in nitric acid. One grain of the dry
salt explodes very violently if heated. It surely is silver azide, AgN3. The yield is however only small: I received in two attempts 5 % and 6,5 % of
the theoretical amount of hydrazoic acid. In the second attempt I washed the benzene solution after the reaction with 20 ml water; perhaps it is to be
attributed to this circumstance that thereby the yield was somewhat bigger. In the benzene solution remains a lot of unchanged nitrogen trichloride.
Better results can be obtained by the reaction of nitrogen trichloride with free hydrazine. I proceed like above described, only with that differences
that I give a 10% sodium hydroxide solution in small portions (3-5 ml) occasionally to the
separating funnel, until the aqueous solution reacts continuously strongly alkalinely. In the whole one 30-35 ml caustic solution is used. The
operation lasts 1 1/2 hours under frequent shaking. Now the aqueous solution is neutralised with sulfuric acid and after the addition of 10 ml of
normal sulfuric acid a quarter of the liquid is destilled over. One time
the distillate did not contain hydrochloric acid, another time only traces of it. Acidimetric (?titration?) and by the weight of the silver salt was
determined that the yield of hydrazoic acid reached 36 % of the theoretical, calculated to 1 g nitrogen
trichloride. From the titrated acid I got the silver salt and determined therein the silver; 71,13-71,03% silver was found,
while silver nitrite is said to contain 71,92% silver.
Because during the reaction of nitrogen trichloride with hydrazine, gases (nitrogen?) and other byproducts are not formed in remarkable quantities, I
believe that the yields of hydrazoic acid will be higher when working with larger batches and that the described synthesis of this very interesting
acid can be used in some cases. During the reaction of potassium
nitrite (Angelo Angeli) or of nitric acid (Sabanejeff) with hydrazine salts form only small quantities of the hydrazoic
acid, only the method of Wislicenus gives better results.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
THANK YOU
 
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The Hodgkinson Azide Patents
There have been many experiments done by me trying to replicate the reactions of the Hodgkinson patents ,
with no success .
It is my opinion that the reaction mechanism simply does not work as described in these patents .
However here they are as attached files for anyone else who may wish to try their luck .
Thanks to franklin for image cleanup and editing
of the pdf's for these two old microfiche patent images .
GB128014 attached
[Edited on 5-1-2008 by Rosco Bodine]
Attachment: Azide from N2H4 + NaNO2 - GB128014.pdf (693kB)
This file has been downloaded 2078 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
GB129152
GB129152 ( attached file )
Thanks to franklin for cleanup and image editing of this
old microfiche image file .
[Edited on 5-1-2008 by Rosco Bodine]
Attachment: AgN3 - GB129152.pdf (652kB)
This file has been downloaded 2756 times
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Pre 1902 Ber. is found at Gallica, I can write and pronounce German with confidence because it is/was suggested to amerikan chemistry students to take
German - but since I have little need to ask where the train station is, what little that I learned in German 1 is useless. I say "schade"
sometimes.
These are the Hentschel refs for NCl3 from the above (otherwise complete) article and Inorganic Syntheses: Ber. 30, 1792-5 and 2642 (1897).
Attachment: hentschel_ncl3.pdf (273kB)
This file has been downloaded 2436 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | Originally posted by halogen
Pardon me, if I have asked before, however; Could dilute hydrazoic acid or ammonium azide not be formed by bubbling N2O through warm ammonia solution,
by the reaction
NH3 + N2O --> H2O + HN3
?
[Edited on 16-4-2005 by halogen] |
See US3012851 for a description of the
reaction and conditions which do work .
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
probably as easy as it gets
JACS 27, 551 (1905):
Attachment: azides_from_h2o2_and_hydrazine_sulfate.pdf (607kB)
This file has been downloaded 2976 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
interesting method has potential ....
However , it sure does involve a lot of handling of bulky dilute solutions , from which is derived a pretty small yield relative to the volume of the
reactants which must be distilled .
Studying the method , it looks like there is the potential there for working with more concentrated solutions and perhaps increasing the yield to a
point where the synthesis would have more value .
Just looking at the charted experiments ,
it seemed to be an interrupted logical progression not to next try 1 gram of hydrazine sulfate per ~20 ml of 3% H2O2 with 5-10 ml conc. H2SO4 and no
H2O .
Then to do a series of followup experiments to examine the reaction under more concentrated conditions ,
in order to discover the optimum economy
for a reasonable reaction mixture volume at which is produced practical yields .
I have a feeling that a good yield might be possible adapting the reaction to be performed in a mixture of 27% H2O2 and
electrolyte grade 1.260 d , ( 35% ) , 4.5 molarity H2SO4 , with some amount of dilution H2O added if necessary .
If the reaction would proceed with as good or better ,
( or at worst not too much reduced yield ) at double or triple the
concentration of the reaction mixture ,
then it would be more practical , for not requiring many liters of distillate to obtain a very few grams of azide .
When I experiment with this method , I will likely discharge the distillate directly
into an alkaline solution , such as perhaps
a cold stirred aqueous suspension of excess Ca(OH)2 ,
producing highly soluble Ca(N3)2 .
When the distillation
is complete to a rising boiling point indication , the solution in the receiver
will be heated to almost boiling and filtered hot to remove unreacted
Ca(OH)2 , taking advantage of its greatly reduced solubility in hot solution . Upon
concentration and evaporation of the filtrate should be obtained crystals of the
trihydrate of calcium azide in relatively pure condition . This could be used in the same way as sodium azide is used in most
syntheses .
Or sodium azide could be obtained by conducting the distillate into a sodium hydroxide or sodium bicarbonate solution ,
slightly less than the amount of theory needed for neutralization of the HN3 to
be distilled , and continuing the introduction of distillate until the solution
indicates a slight acidity . Concentration and evaporation of the solution would give pure sodium azide .
Actually this production of the sodium azide could be done first , using quantities
of alkali certain to be neutralized by the
expected yield of HN3 in the first three quarters of the distillate . And when the
solution of sodium azide is complete and
it begins to acidify with free HN3 from the remaining distillate , then the remaining distillate could be discharged into the cold limewater to
safely absorb the remaining HN3 still coming over , and provide a method of its recovery in usable form .
|
|
|
Lambda
National Hazard
   
Posts: 566
Registered: 15-4-2005
Location: Netherlands
Member Is Offline
Mood: Euforic Online
|
|
A source of Barium Azide
OTC AZIDES ? Originaly posted by Rogue Chemist on 8-2-2005
Barium azide is used in fluorecent tubes as a frequency converter. The original source being ultraviolet radiation caused by ionized "MERCURY
VAPOUR". These TL tubes are often coated by mixtures of flourecent salts, and depending on the manufacturer have a specific color code and
composition. The pop that you hear when rupturing a TL tube is an implosion. The real hazard involving tube rupture is the mercury vapour, and an
implosion often tends to give larger chunks of flying glass as would be expected with an explosion. Wounds caused by this stuff can be slow healing
and cause intoxication.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Distilling HN<sub>3</sub>? I would certainly stay away from that, as the gas is extremely poisonous (much more so than HCN).
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
But I bet you would work with nitrations routinely where nitric oxide is but little less deadly .
When you do such a distillation , it is easily done as a closed but vented system process . Most of the HN3 will
likely come over in the early part of the distillation and in diluted aqueous form
through a cold condenser , being discharged directly into a neutralizer which
ties up the material as a nonvolatile salt ,
while any unreacted fumes are vented away through a length of tubing and discharged into the air at a distance or
aspirated down the drain in a water stream . It's really quite simple to set up
the apparatus to do this safely .
But in chemistry it is sort of like driving a car , careless mistakes can kill you , and so can not knowing what you are doing ,
or just plain bad luck , and not necessarily
in that order .
|
|
|
| Pages:
1
2
3
4
5
..
18 |