| Pages:
1
2
3 |
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by mr.crow  | | After leaving the CuCl2 in a desiccator for many weeks with NaOH and CaCl2 the small green crystals turned light blue. Success!
|
failure! i left my CuCl2 solution on a large petri dish in a cool room to dry. as previously mentioned, the stuff smells acidic, so i guess
the hydrated chloride hydrolyzes and gives off HCl even in the cold. the leftover must be basic, probably Cu2(OH)3Cl. indeed, today i noticed some of the crystals had turned light blue. i was reminded of your post and reckoned you must have been using
a base as a desiccant (otherwise the HCl would soon have reached an equilibrium in the closed space of a desiccator, and the decomposition of the
CuCl2 would have stopped). my conclusion proved correct.
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
I am somewhat skeptical of that analysis of the results. According to this study on the hydrolysis of copper ions, *β1 = 7.7 x 10^-9 and *β2 = 2.1 x 10^-17, where *β represents the equilibrium constant
of the hydrolysis. Compare this to the *β1 of iron (III), which this article claims is 10^(−2.18 ± 0.01). I have made copper chloride previously, and they turned out green due to formation of
CuCl4-2 ions. Some time later, after leaving them out, they turned blue, which I attributed to a return to CuCl2 as opposed to more complex
tetrachlorocuprates. Moreover, with much less access to water, the hydrolysis of copper is expected be lower as a hydrated salt than for a solution.
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
that might explain the color change, but not the HCl given off, which necessarily leads to a stoichiometric imbalance between copper and chloride ions
in the residue.
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
After reading up on the thread again, I found woelen's post that contained this line:
| Quote: | | The grass-green compound is an acidic compound, which contains HCl as well. It contains complexes like HCuCl3.xH2O and these cause the green color.
|
This implies that the crystals themselves are not hydrolyzing, but rather, giving off HCl already contained within them, and in doing so, forming the
blue CuCl2 sans tri- and tetrachlorocuprate complexes. The HCl would have been formed by the hydrolysis of copper in solution. Assuming
[Cu+2] is at a maximum, according to the *β1 and *β2 given above, around 0.0021 M of HCl would be formed, and the hydrolyzed
copper components precipitated. They would have been filtered or contained within the green copper chloride crystals. The small amount of HCl produced
by the hydrolysis accounts for the acidic smell, since the odor threshold of HCl is only about 7 mg/m3.
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
thanks for pointing that out, that explains many things. BTW can anhydrous copper sulfate precipitate from aqueous solutions?
my product has lost the smell and is now turquoise:

a closer look shows distinct crystal species admixed, namely the colored CuCl2.2H2O needles plus clear NaCl cubes (no noticeable
sulfate crystals):

|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
I would be quite nonplussed if anhydrous copper sulfate precipitated from an aqueous solution. Double salts? Sure. But not anhydrous forms of salts
that have hydrates.
As for the sodium contamination, that's pretty par for the course. From what I read on this forum, it's astoundingly difficult to completely remove
sodium contamination. It's one of the benefits of using copper carbonate to make soluble copper salts, since sodium contamination tends to be lower,
provided that the CuCO3 is washed well.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I would suppose CuSO4 can be grown from a high temperature solution (i.e., in an autoclave), since the decomposition point of CuSO4.5H2O is relatively
high. Compare to NaSO4.10H2O, which melts at 80C or so and therefore the anhydride can be grown from solution at atmospheric pressure.
Concerning mixtures: I seem to recall I have a baggie of about a pound of undefined copper mixture. IIRC, it seems to have small sulfate crystals,
lots of chloride, probably sodium chloride as well, and maybe others. I recall also I had dried this material over paper towels, which partially
decomposed due to the acidity, and I assume the cellulose fragments (including some glucose) reduced some copper, putting brown Cu2O crystals into the
mix as well! I suppose some day I should dissolve it, dump in Na2CO3 and do the carbonate workup.
Tim
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
turns out that CuCl2 is very soluble in ethanol, NaCl only slightly, whereas the sulfates not at all. this is what the product looks like
after recrystallization from warm 95% EtOH (in shades of grey):
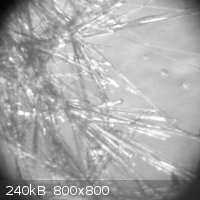
the needles formed several star-shaped centers on the petri dish. the ethanolic solution was again intensely green, yet no HCl smell was observed. the
insoluble sediment gives only a pale blue solution when dissolved in water, a sign that most copper was removed. no reduction to the monovalent cation
was noticed. note that some NaCl passes through the filter and falls out upon cooling; this was removed by decantation.
the desire to remove all sodium is usually confined to applications in pyrotechnics. a bit of contamination is no big deal, as long as it's not so
blatant as in the crude product.
|
|
|
Vargouille
Hazard to Others
  
Posts: 380
Registered: 16-4-2012
Member Is Offline
Mood: No Mood
|
|
When purifying copper compounds, I use bicarbonate, since there's not much hydroxide formed, and copper bicarbonate decomposes into copper carbonate.
When you use carbonate, you get a mixture of hydroxide and carbonate. If you use it immediately and you don't mind the uncertainty, it doesn't matter,
but since I rarely use mine immediately, I dislike the uncertainty of the inclusion of hydroxide and oxide, from the decomposition of hydroxide, I use
bicarbonate.
|
|
|
| Pages:
1
2
3 |