| Pages:
1
2
3
4 |
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Making a primary amine
The reaction happens in two stages. In the first stage, a salt is formed - in this case, ethylammonium bromide. This is just like ammonium bromide,
except that one of the hydrogens in the ammonium ion is replaced by an ethyl group.
There is then the possibility of a reversible reaction between this salt and excess ammonia in the mixture.
What do you actually get if you react bromoethane with ammonia?
Whatever you do, you get a mixture of all of the products (including the various amines and their salts) shown on this page.
To get mainly the quaternary ammonium salt, you can use a large excess of bromoethane. If you look at the reactions going on, each one needs
additional bromoethane. If you provide enough, then the chances are that the reaction will go to completion, given enough time.
On the other hand, if you use a very large excess of ammonia, the chances are always greatest that a bromoethane molecule will hit an ammonia molecule
rather than one of the amines being formed. That will help to prevent the formation of secondary (etc) amines - although it won't stop it entirely.
Basically use as much ammonia as possible or a very large excess of ammonia and react with bromoethane. I don't know much of the details or conditions
of the reaction
The ammonia removes a hydrogen ion from the ethylammonium ion to leave a primary amine - ethylamine.
The more ammonia there is in the mixture, the more the forward reaction is favoured.
--------------------------------------------------------------------------------
Note: You will find considerable disagreement in textbooks and other sources about the exact nature of the products in this reaction. Some of the
information you'll come across is simply wrong!
You can read the arguments about the products of this reaction by following this link.
Warning! That page is in the mechanism section of the site. Return to the current page using the BACK button on your browser. If you use the links at
the bottom of that page, you could get seriously lost!
--------------------------------------------------------------------------------
Making a secondary amine
The reaction doesn't stop at a primary amine. The ethylamine also reacts with bromoethane - in the same two stages as before.
In the first stage, you get a salt formed - this time, diethylammonium bromide. Think of this as ammonium bromide with two hydrogens replaced by ethyl
groups.
There is again the possibility of a reversible reaction between this salt and excess ammonia in the mixture.
The ammonia removes a hydrogen ion from the diethylammonium ion to leave a secondary amine - diethylamine. A secondary amine is one which has two
alkyl groups attached to the nitrogen.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Wait! There's more!
What do you actually get if you react bromoethane with ammonia?
Whatever you do, you get a mixture of all of the products (including the various amines and their salts) shown on this page.
To get mainly the quaternary ammonium salt, you can use a large excess of bromoethane. If you look at the reactions going on, each one needs
additional bromoethane. If you provide enough, then the chances are that the reaction will go to completion, given enough time.
On the other hand, if you use a very large excess of ammonia, the chances are always greatest that a bromoethane molecule will hit an ammonia molecule
rather than one of the amines being formed. That will help to prevent the formation of secondary (etc) amines - although it won't stop it entirely.
Basically, use a large excess of ammonia and react it to bromoethane. I don't know much of the details of the reaction.
http://www.chemguide.co.uk/organicprops/amines/preparation.h...


|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Hooray for ethylamine!

|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Methyl Ethyl Ketone ----haloform---> sodium (calcium) propionate -----> propionic acid ---urea----> propanamide ----Hofmann
rearrangement---> ethylamine
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Can ethylamine be obtained from reacting hydroxylamine with ethyllithium?
"Imagination is more important than knowledge" ~Einstein
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Acetaldehyde reacts with Hydroxylamine to produce ethylidenehydroxylamine.
CH3CHO + NH2OH ==> CH3CH=NOH
It might have nothing to do with ethylamine though. 
"Imagination is more important than knowledge" ~Einstein
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Jeeze, buy some Platinum, produce some catalyst, and hydrogenate Acetonitrile.
Some of the guys get gallons of Acetonitrile for free. Under some conditions, Diethylamine may be produced, when the imine intermediate condenses
with already formed Ethylamine.
I'll try to produce a reference.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Quote: Originally posted by zed  | Jeeze, buy some Platinum, produce some catalyst, and hydrogenate Acetonitrile.
Some of the guys get gallons of Acetonitrile for free. Under some conditions, Diethylamine may be produced, when the imine intermediate condenses
with already formed Ethylamine.
I'll try to produce a reference. |
I think that may involve having the reactants in the gas phase.
"Imagination is more important than knowledge" ~Einstein
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
What about ethylamine dissolved in acetonitrile and using an amalgamation type reaction with Al/Mg (Aluminum and Mercury). 
"Imagination is more important than knowledge" ~Einstein
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
LXXXII.—The preparation of ethylamine and of diethylamine
Emil Alphonse Werner
J. Chem. Soc., Trans., 1918, 113, 899-902.
DOI: 10.1039/CT9181300899
Attachment: The preparation of ethylamine and of diethylamine.pdf (136kB)
This file has been downloaded 1248 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
I don't have access to the full article, but I was thinking of something more along these lines.
http://www.deepdyve.com/lp/elsevier/liquid-phase-hydrogenati...
www.springerlink.com/content/t2004w4m2418n779/
https://docs.google.com/viewer?a=v&q=cache:CYh-nxTSnJEJ:...
[Edited on 30-9-2012 by zed]
[Edited on 30-9-2012 by zed]
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Maybe ethylamine can be obtained via ethanol and a tosyl chloride intermediate. Then reacting the intermediate with ammonia. Maybe there will be
ethylamine without diethylamine or triethylamine. 
Here's the reference.
http://www.erowid.org/archive/rhodium/chemistry/mdma.tosylat...

"Imagination is more important than knowledge" ~Einstein
|
|
|
triskelion
Harmless

Posts: 2
Registered: 6-10-2012
Member Is Offline
Mood: No Mood
|
|
Try the decarboxilation of alanine in high boiling solvent with ketone catalyst.
In this article, the authors made it with tryptophan obtaining tryptamine:
S. Takano, T. Nishimura, K. Ogasawara "Heterocycles 6(8), 1167-71 (1977) ".
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Decarboxylation of amino acids
| Quote: |
Methylamine from glycine
10 g glycine and 15 g urethane (molar excess) were dissolved in 50 ml 0.25M Ba(OH)2 and heated for 2 hours. After precipitating the baryta as the
carbonate by passing CO2 through the solution, the filtrate was acidified with a couple of drops of HCl and the solution was evaporated. The hydantoin
was not isolated, yet 50 ml conc HCl was added and this was refluxed for 10 hours, after which the major part of the solution was evaporated.
This caused the hydantoin to completely transform into methylamine, so that by addition of ethanol no hydantoin was precipitated, and the
characteristic reaction (2) of amines with sodium nitroprusside and acetone was obtained.
The solution was decolorised with animal charcoal and picric acid was added. After evaporation the picrate was obtained as brightly yellow plates with
mp 215°C. Yield: 29 g picrate (84%)
M. Wada - Biochem. Z. 260, 47, 1933
http://www.sciencemadness.org/talk/viewthread.php?tid=9915&a...
|
I think replacing alanine with glycine should lead to Ethylamine
[Edited on 15-10-2012 by Waffles SS]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
OK, I'd probably try hydrogenating acetonitrile over CoB on Carbon, in Isopropyl alcohol saturated with NH3.
Worked extremely well for reducing Propionitrile to Propylamine. As in example 7.29
http://www.scribd.com/doc/73915503/Handbook-of-Heterogeneous...
The whole of "The Handbook for Heterogeneous Hydrogenation for Organic Synthesis" , now seems to be available online, for free perusal. Courtesy of
Wiley.
[Edited on 20-10-2012 by zed]
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Ammonium chloride condenses with formaldehyde to form methyleneimine. Can a grignard reaction be done with the grignard reagent of methyl chloride to
form ethylamine?
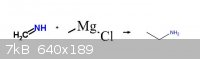
[Edited on 26-10-2012 by ChemistryGhost]
"Imagination is more important than knowledge" ~Einstein
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Seriously? Please give us a reference for a preparative reaction of methyleneimine.
|
|
|
triskelion
Harmless

Posts: 2
Registered: 6-10-2012
Member Is Offline
Mood: No Mood
|
|
to chemistryghost:
the reaction between formaline and ammonium chloride goes on and lead to methyl, dimethyl, trimethylamine hydrochloride.
The Grignard reaction to methyleneimine should works, but it's very difficult to obtain it.
Impossible by the reaction you proposed.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, I don't like your chances. Polymerization is more likely, and of course the H20 formed via the condensation of formaldehyde and ammonia, is
probably more reactive than the imine.
In fact, the DOJ has reported regrets that such reactions usually do not work.
During several raids on novice labs trying to produce Methamphetamine, they found zero product.
The reaction sequence of condensation of Acetaldehyde with Methylamine, followed by Sodium Sulfate drying, and reaction with Benzyl-Magnesium
Chloride....Does not work well.
More stable imines can reportedly be used in such reactions,
Methyleneimine is probably not a good prospect.
|
|
|
Sydenhams chorea
Harmless

Posts: 29
Registered: 16-8-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Waffles SS  |
Decarboxylation of amino acids
| Quote: |
Methylamine from glycine
10 g glycine and 15 g urethane (molar excess) were dissolved in 50 ml 0.25M Ba(OH)2 and heated for 2 hours. After precipitating the baryta as the
carbonate by passing CO2 through the solution, the filtrate was acidified with a couple of drops of HCl and the solution was evaporated. The hydantoin
was not isolated, yet 50 ml conc HCl was added and this was refluxed for 10 hours, after which the major part of the solution was evaporated.
This caused the hydantoin to completely transform into methylamine, so that by addition of ethanol no hydantoin was precipitated, and the
characteristic reaction (2) of amines with sodium nitroprusside and acetone was obtained.
The solution was decolorised with animal charcoal and picric acid was added. After evaporation the picrate was obtained as brightly yellow plates with
mp 215°C. Yield: 29 g picrate (84%)
M. Wada - Biochem. Z. 260, 47, 1933
http://www.sciencemadness.org/talk/viewthread.php?tid=9915&a...
|
I think replacing alanine with glycine should lead to Ethylamine
[Edited on 15-10-2012 by Waffles SS] |
I think not. Had you used the search engine, you would have found that the Wada article is complete bullshit, and that hydrolysis of the hydrantoin will just
give back the original amino acid.
link to the pdf: An Investigation of Wada’s method of Converting alpha-Amino acids into 2-Substituted Ethylamines
| Quote: | | The reaction (Wada, Biochem. Z., 1933, 260, 4T), whereby an a-amino-acid is converted into a 2-substituted ethylamine by hydrolytic decarboxylation of
the corresponding hydantoin, could not be repeated: the amino-acid was regenerated in each case. Some observations on the Rimini reaction for primary
amines are recorded. |
Il n'y a point de sots si incommodes que ceux qui ont de l'esprit.
François de La Rochefoucauld.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
@Sydenhams chorea ,
You are right,it seems this is bullshit article
But what about :
Amino Acid DecarboxylationCatalyzed by 2-Cyclohexen-1-One
http://www.erowid.org/archive/rhodium/chemistry/trp.decarbox...
This is bullshit too?
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
I think propionitrile hydration with hydrochloric acid to propionamide, then sodium hypochlorite Hoffman rearrangement to ethylamine could work.
"Imagination is more important than knowledge" ~Einstein
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Feasible but your source of propionitrile is..?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by ChemistryGhost  | | I think propionitrile hydration with hydrochloric acid to propionamide, then sodium hypochlorite Hoffman rearrangement to ethylamine could work.
|
You again with the proposing of noneconomical routes, Blue Dex?
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
ok so- in my hunt for ethylamine, - chloroethane
"The advantage of use of an emulsifying agent is shown by shaking an alkyl clfioride, say ethylene chloride, with 19% ammonia solution. Where no
emulsifying agent is used the heavy chloride separates to form a colorless layer within half a minute but Where a small amount of ammonium oleate is
present no such separation occurs until after many ,hours."
https://patents.google.com/patent/US2113640
"Thus, about 20 parts ethylene dichloride, containing small amounts of oleic acid, for example, 0.5% (or 0.1 part by volume after aqua ammonia has
been added) which will give an ammonium soap"
they mention.. 10psi to 300 may be used, 10 psi is 0.68 bar, so below atmospheric pressure? does this reaction form gasses- could it maybe be put in a
plastic bottle and just be contained for a week to keep it a slight bit pressurized?
"The reaction is effected at a temperature of about 100 C. to about 200 C. and preferably about 125 C. and is about complete in about 40 minutes at
about 125 pounds per square inch gauge pressure."
for every 10*C reaction speed is doubled, 40 minutes.
can we assume it will actually happen at room temperature?
i did also have success with theanine- i just mixed that up with 20% H2SO4 and reacted at maybe 60*C for 6 hours or so, then NaOH into that, theanine
is rather costly however, 100g cost me about 20 euros or was it just 10?
it was same price for 250g alanine - as im attempting to turn it into nitroethane i tried briefly to oxidize alanine with H2CrO4, no luck it seemed -
amine to nitro is typically done with manganate of even KHSO5+acetone
anyhow the alanine was reacted at about 160*C with 1g copper chromite per 50g, it was powdered well first. this however turns into a rather diabolic
chunk in a glass flask, HCl seems to take care of it, it seems HCl must then be reacted with NaOH to free more amine from the mixture- but also to
free the bottle- high boiling solvent can maybe fix this?
some amine was acquired from the decarboxylation of alanine with copper chromite to assist it, milk heated with NaOH and suspected amine will
typically tell if you have an amine, methylamine turned the mixture pink after some minutes of heating, ammonia wont do this. iirc it turns brown or
yellow instead?
|
|
|
| Pages:
1
2
3
4 |