| Pages:
1
2
3
4
5
..
10 |
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Sugestion:
...................Thermostat
------------------------|..|---------------------
Refractory mix......|..|
-------------------------|..|--------------------
C. w. heating coil..|..|
========================================= space for dilatation
---> Exhaust flow, pipe length approx. 30cm
========================================= space for dilatation
Concrete with heating coil
-----------------------------------------
Refractory mix
-----------------------------------------
lousy drawing, but the idea is to insert the thermostat tube vertically, somewhere along the tube.
I think you shold give your glass tube space to dilatate.
If you want to give your NiCr wire space to dilatate also , first give it a bath of molten paraffin, not very hot. This will create a layer of
paraffin around your wire. Than embed it in phosphate cement. This will make a hellish stench the first time you heat it, but after the paraffin has
disapeared (I don´t know if it evaporates, disintegrate or whatever, but I know it works, I´ve done it) Your wire will have space to diltate. I have
made embedded heating elements without this procedure and they work OK, but I thought I should give you the idea, you will be investing a lot in it.
[Edited on 11-3-2004 by Tacho]
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Good idea. One modification though: I'll use stearin instead of paraffin, since I've got plenty of candles but no paraffin... 
Edit: More progress has been made:
http://species8472.dyndns.org/so3/so3.html
[Edited on 2004-3-12 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Oooooh! The first Macintosh browser to ever have accessed my webserver has accessed it. Interesting.
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
I just tested the thermostat again and its is fine. Humidity is no problem at all.
Use thin electrodes. The heating of a thick wire takes long because it acts as a dissipator of heat. Causes slow response. Mine are 1,5mm2 and I
noticed that effect.
Also, I just washed the test tubes I used for inicial tests and it seem the molten salt does not attack the glass at all.
How is the project?
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Progress.
It's progressing nicely. I'm basically just waiting for the concrete/bentonite mix in the burner bottom to fully harden, then I'm going
to cast the top and construct the heater (again, you know what happened to the last one... fscking decimal signs.....).
I have bad experience dehydrating concrete/bentonite mix before it's fully hardened, hence the long waiting period.
I'm still not sure whether to have the NiCr coil exposed to SO2 vapors or to insulate it someway. I *think* that provided no water is introduced
(eg. I'm going to use an air dessicator after the air pump), the NiCr wire won't get attacked. That would be the simplest way. Just a coil
of 0.2mm dia NiCr wire inside a glass tube, controlled by a dimmer and using your salt thermostat idea as an indicator. But I could be wrong. And
right now, I'm drunk as well. Perhaps it shows.
As previously said, I will construct this plant in an extremely modular way --- if one part fails, I'll just need to replace it.

But regardless, it's very exciting to downscale an industrial process by a factor of 1000. My goal is to make the entire plant fit on a 500x500
mm table. Then, when it's done, I'll cover the whole table with a fume hood and an air exhaust, just to be on the safe side.
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
scaledown
Axehandle you are very right. You are doing the opposite of scaleup - a common task of chemical engineers. In fact there is a whole field devoted to
this called dimensional similitude, like using models in wind tunnels, etc.
An anecdote: I once had a job to figure out how to get a 1 gallon lab corn starch converter to behave like a 3000 gallon plant production converter.
The process consisted of "cooking" a water/starch slurry with an enzyme for about 2 hours. Of course I started using the same cooking time
and same recipe. But the production size unit gave a higher conversion. I tried varying the recipe and agitator tip speed but never could duplicate
the plant results. It probably could have been done I just gave up as it was taking too much time.
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
So I hacked together a sulfur burner today. Alas, the sulfur I got at the DIY warehouse ("Green Light" wettable dusting sulfur) is quite
impure. It created huge masses of smoke and left large quantities of clinker in the device. The run was not succesfull. I'll make another go
tomorrow, see if the impurities settle out of a melt.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Geomancer,
Are you trying to make a small sulfuric acid plant like axehandle's or just experimenting with sulfur?
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
I'm very sorry for your contaminated burner.
I get my sulfur in a store that sells mined sulfur from Sicily. It's quite pure. Another option would be sublimed sulfur ("flowers of
sulfur" available anywhere. But there will always be small contaminants. available anywhere. But there will always be small contaminants.
I will insert a wash bottle after the burner to get rid of solid particles. Wouldn't want to poison my catalyst, I've invested far too much
time in it....
Edit: I'd get a purer kind of sulfur, I've read that gardening sulfur contains up to 10% impurities.
[Edited on 2004-3-14 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
I'm interested in the technical problems of the small scale contact process. It remains to be seen if I'll ever build a complete plant.
I think I will forgo any further experimentation with my current sulfur. Does anyone have good suggestions for sulfur sources (in the range of a
few pounds) in the eastern US?
[Edit: I tried burning a nice yellowish chunk of sulfur that froze in the burner in open air. It ignited vigourously, burning with a blue flame, but
quickly turned black and self-extinguished. When I scraped up the residue (sticky black stuff covered by a crunchy ash layer) and molded it into a
ball, it once again burnt vigourously, charred, and went out. I was able to repeat the process several times.]
[Edited on 15-3-2004 by Geomancer]
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
This is just idle speculation, but I'd think you'd be able to refine the sulfur by melting and sedimentation. Oop! Reminds me, time to turn
off the oven. The sulfur burner bottom half should be done now. Tomorrow I'll cast the lid!
Edit: And I will take a lot of pictures. If the design works, it's probably the cheapest and most efficient burner I've ever seen. If it
doesn't --- well, I've only made a fool of myself. I'm quite used to that by now...
[Edited on 2004-3-15 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
It should be possible to clean sulfur in the same way red phosphorus is cleaned: Washing with lots of water, dilute acids, water again, dilute NaOH
solution and cupious amounts of distilled water at the end.
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
When I die, I'm going to sue both axehandle and Green Light  . I placed a
sizeable amount of the dusting sulfur into a pan on a hot plate. At first, the heated sulfur became slightly more yellow. As it began to melt, there
was a small amount of bubbling and the liquid in contact with the pan started to turn reddish. As the entire mass melted, the bubbling became
vigorous, turning the mass into a watery foam, somewhere between the color of dog vomit at the top and brown sugar below. . I placed a
sizeable amount of the dusting sulfur into a pan on a hot plate. At first, the heated sulfur became slightly more yellow. As it began to melt, there
was a small amount of bubbling and the liquid in contact with the pan started to turn reddish. As the entire mass melted, the bubbling became
vigorous, turning the mass into a watery foam, somewhere between the color of dog vomit at the top and brown sugar below.
I'm not sure what the evolved gas was. According to my mother, it doesn't smell like rotten eggs, so hopefully it's something less
toxic than H2S. It definately smelled "sulfurous", but it didn't burn by eyes like I would expect SO2 to. Moreover, white smoke
(perhaps condensation from the air, though it's not that humid here) also evolved at the hieght of the reaction. The only suitable vessel I could
find was an aluminum pan. It may have been involved, but I think not. The mixture took a while to cool, and retained a low viscosity up to the point
where it froze. Also, gas evolution continued at a diminished pace even after the upper layer had solidified.
What is it that makes this sulfur so evil?
What was the gas that was formed?
Will I survive?
Inquiring minds want to know.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Oh dear. I didn't mean "on a hot plate"!!! My theory is that you got "hot spots" that lead to oxidation of some of the
sulfur, yielding SO2. Don't worry, it's only lethal in 1000ppm++ amounts. There's no way you made H2S, at least not that I'm aware
of.
What about using Organikum's method instead?
On a related note: You can't sue me. In my country we have laws that basically say that "whatever you've read or heard, you've
only yourself to blame for taking the advise..." 
I'm very sorry for not being more clear, though. Using hotplates together with combustible substances is suicidal. I'm sorry for not being
more clear. Why not try melting the sulfur in a more controlled manner, like, say, in your kitchen oven?
Edit: Btw, SO2 in small amounts doesn't "burn your eyes" in my experience. It only leads to a bad cough. I know from a little accident
I had that taught me some valuable lessons.
Edit2: I would blame the contaminants in the sulfur for the gas development after you stopped heating it. It could also be sublimed sulfur. When I
tried to melt sulfur in a pan (!) in my oven, the damned thing got covered on the entire inside with sublimed sulfur. Took me 300C at 3 hours to burn
it off.. use a somewhat closed vessel, like an E-flask. Then you can let it settle and pour the molten sulfur into cold water. Then wash with, say,
acetic acid and then distilled water, just like Organikum said. But if I were you, I'd simply buy some purer sulfur...
[Edited on 2004-3-15 by axehandle]
[Edited on 2004-3-15 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
I suspect that if I used the kitchen oven, my house would be full of the unknown gas. Ovens have poor temperature control, and the knobs are badly
calibrated. Moreover, my oven is a gas oven, and so uses an open flame. I cannot discount the hot spot theory, but it seems unlikely that the
temperature would be very much over that desired while there was still solid sulfur present. Moreover, very hot liquid sulfur becomes more viscous. My
stuff remained watery the whole time. I also don't think that atmospheric oxygen was responsible. I just don't see that route causing
foaming. While better temperature control would help settle things, I'm not going to try it without either a compelling reason or better
information as to what's in this witches brew.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
For pete's sake mate, DON'T discount the hot spots! That's how I almost blew my face off melting rocket fuel on the stove!!!!!!
Believe me, hot-spots are BAD!!!!
Edit: That said, buy some purer sulfur. It should be called "flowers of sulfur", i.e. sublimed sulfur, quite pure.
[Edited on 2004-3-16 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Now the bottom is dehydrated. Stupidly enougn I'll have to wet the bottom of the burner hole, since that's where I will place a heating coil
embedded into phosphate bonded cement. I have spun a coil of 0.3mm dia NiCr wire, resistance adjusted to a 300W dimmer.
I've learnt that sulfur has a boiling point of 444C, and an auto-ignition temperature of 248-266C. Hence there shouldn't be any need of an
ignition device, heating it to the auto-ignition temperature and then holding that temperature while providing oxygen should suffice.
Now, the tricky part will be the connectors. They'll be in contact with burning sulfur in a molten state. What material? I'll have to think.
The logical choice of material would be gold-plated nickel. I'll check up the prices.
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Sulfur in the molten state is actually quite corrosive. I read about it extensively when I was reading about carbon disulfide production. The
molten/vaporized sulfur would come into contact with the heavily heated carbon and react. And guess what they use to coat the vessel? ALUMINUM!
That has the incredibly high resistance to sulfur that one would dream of, however for the carbon disulfide reaction it wasn't very useful
because at the temperatures employed for the vaporized sulfur to get the highest yield the aluminum would be molten. The papers went on to descibe
the properties of high aluminum alloys to use in place of straight aluiminum to run at higher temperatures.
Also, Geomancer, the color changes and changes in viscosity were normal except maybe that last one where it turned like dog vomit. It is because the
sulfur rings S8 break apart at the higher temperatures and form polymer sulfur (which you can pour in cool water and get plastic sulfur from) then at
higher temperatures it breaks apart and changes color and texture again. Finally at exceedingly high temperature it boils and makes S2 molecules, S2
is green I think, could be wrong. Also, did you know that the alkali metal vapors are brightly colored, I'm pretty sure gaseous potassium is
green too.
[Edited on 3/16/2004 by BromicAcid]
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Great! I'll just cut up a piece of aluminum pipe lengthwise to obtain 2 Al strips then. The temperature I anticipate in the burner will never
exceed 400C, far below the 622C melting point of aluminum. Thanks.
Edit: It also means that I don't have to make the lid detachable! All that's needed is one 20mm aluminum pipe for sulfur addition, another
one for a future temperature probe, sealed during burning with 2 soft-lead plugs! Halleluja!
Edit2: Thanks to my rocket hobby I've got tons of aluminum pipe! Oh, yes, precious...
[Edited on 2004-3-16 by axehandle]
[Edited on 2004-3-16 by axehandle]
[Edited on 2004-3-16 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
Bromic: There was no change in viscosity. It was very fluid at all times.
It rained today. The solidified mass has turned to slush. Apparently, whatever made the stuff wettable concentrated at crystal boundries, and
dissolved. From what I remember, the melt did not seem to have multiple condensed phases, although the foaming that was going on made it hard to tell.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Ooooh.... My burner's melting sulfur.... I love the smell of brimstone. Let's see if it catches flame when the heating coil embedded in
phosphate bonded cement manages to heat it to the auto ignition point....
Then to the impurities point. How to clean the exhaust from solid particles before it enters the exhaust heater (before the SO2 enters the catalyst
chamber)? Ideas, anyone? Myself, I'm thinking a bubbler. But the water would heat up quickly. I'd prefer a replaceable filter. Would
rockwool do it?
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
de-entrainment equipment
The usual industrial way to remove particles in a gas stream is with a filter, demister pad, and/or sheet metal chevrons, depending on the
particulates and the degree of removal required.
An industrial evaporator I worked on cleaned high velocity steam with a chevron pad followed by a demister pad (like a brillo pad). This was all up
flow so the particles became entrained in condensate which then just drained back into the evaporator.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
New burner design.
Thanks. I think a filter will be simplest, and the easiest to replace.
Right now I'm trying to tackle the sulfur burner problem. I'm not happy with the current design. In fact, I'm very uphappy about it.
I'm gonna scrap the old design and use a completely new one I came up with today.
I'm attaching it, since BB code is disabled here.
Explanation:
The burner is made of cast aluminium (white), with a number of thick rods protruding from the bottom into the combustion/melting chamber to distribute
the heat evenly. Aluminum has excellent heat conductivity.
A simple circuit using the embedded thermocouple (red), essentially a thermostat, controls the heating mantle (blue) so that the pure, elemental
sulfur (yellow) is kept in a molten state close to its auto-ignition point.
Dry, pure oxygen is fed very slowly through one of the pipe-like entries at the top of the Al chamber. As SO2 is formed, it's forced out the
other opening, where it will be passed through a thick rockwool filter. Then it is led into a SO2 heating pipe, and then passed through the catalyst
where it will get converted to SO3, after which it's led to a bubbler with water, where it will form H2SO4(aq).
The heating mantle could be replaced with a hot air gun blowing at the aluminium vessel's side, if a cheaper, more noisy solution is called for.
Now, I know that aluminium is resistant to molten sulfur, as well as to SO2. It should also be resistant to dry O2. However, I'm not sure what
happens when it's exposed to <b>burning</b> molten sulfur combined with O2 and SO2.
It could be that the combustion temperature is higher than the MP of Al, and then my design is seriously fucked.
Anyone please care to give me some feedback?
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Damned. Here is the attachment:
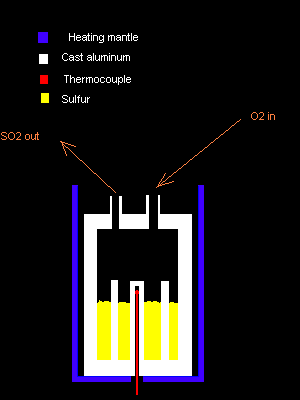
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
rikkitikkitavi
Hazard to Others
  
Posts: 192
Registered: 17-6-2002
Member Is Offline
Mood: No Mood
|
|
axehandle , are you going to use 100 % oxygen as a feed?
imagine sulfur burning in a 100 % oxygen atmoshpere? try it with red-glowing steel wool...
It will be an extremely short buring, when all the oxygen inside the burning chamber is consumed quickly, and the temperature of the SO2 will be very
high ( +1000K). You can calculate the energy released by the heat of reaction and the amount of O2 inside the chamber (296 kJ/mol)
Howver the large aluminium can will probably absorb the heat (if you burn 2 moles/h it equals 150W of power)
Also the auto ignition point of sulfur is increased a lot if SO2 is present , according to Ullmann.
/rickard
|
|
|
| Pages:
1
2
3
4
5
..
10 |